Fig. 3. Roles of molecular chaperone gp96 in oncogenesis from the vantage point of immunology.
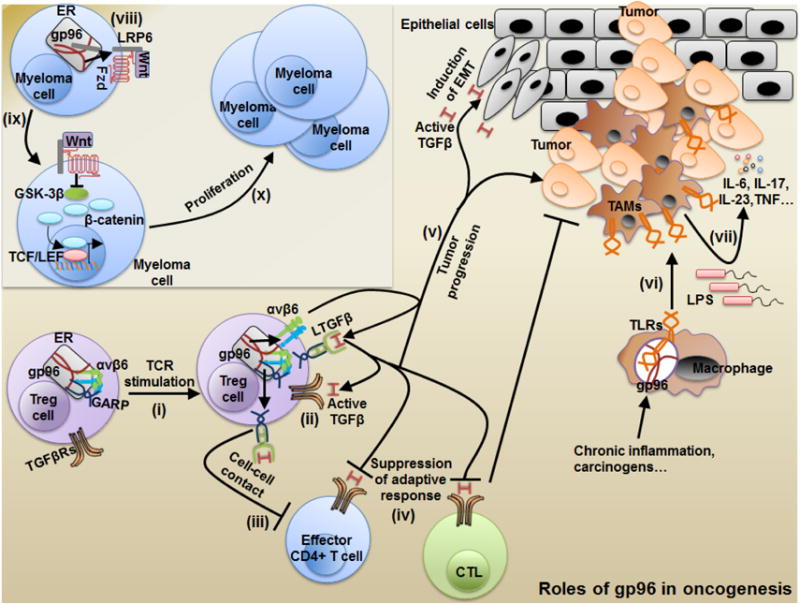
Upon TCR engagement in regulatory T cells (Tregs), gp96 mediates the surface expression of the latent TGF-β docking receptor, GARP (i). Expression of surface GARP provides a site for further binding and activation of latent TGF-β via multiple mechanisms including, cleavage by integrins such as αvβ6 integrins, which are clients of gp96 (ii). Tregs then mediate suppression of effector CD4+ and CD8+ T cells via cell-cell contact using membrane-bound active TGF-β (iii), or soluble active TGF-β released upon integrin cleavage of latent TGF-β from GARP (iv). Treg suppression of the adaptive immune response promotes tumor progression and the release of active TGF-β mediates epithelial-mesenchymal transition of normal epithelial cells (v). Furthermore in macrophages, chronic inflammation coupled with carcinogens triggers gp96 upregulation, which in-turn chaperones TLRs and augments the induction of tumor-associated macrophages (TAMs) (vi). TAMs support tumor progression by releasing cytokines including IL-6, IL-17, IL-23 and TNFα (vii). In addition, gp96 plays a major role in the oncogenesis of multiple myeloma (viii). Gp96 chaperones the Wnt signaling co-factor, LRP6, to the cell surface and this augments Wnt signaling to inhibit GSK-3β and mediate nuclear translocation of β-catenin (ix). This mechanism promotes excessive proliferation of multiple myeloma cells (x).
