Figure 3. Src is required for PDHA1 tyrosine phosphorylation in cancer cells and directly phosphorylates PDHA1 at Y289.
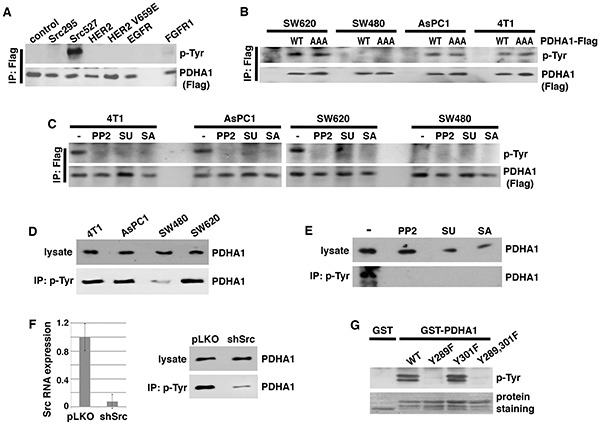
A. Src induces PDHA1 tyrosine phosphorylation. PDHA1 with a carboxyl-terminal Flag tag (PDHA1-Flag) was transiently transfected into HEK293 cells together with indicated tyrosine kinases. PDHA1 was immunoprecipitated with anti-Flag antibodies and immunoblotted with anti-phosphotyrosine (p-Tyr) antibodies. B. Exogenous PDHA1 is tyrosine-phosphorylated in cancer cells. Indicated cancer cells were transduced with lentivirus expressing wild type (WT) or AAA mutant PDHA1-Flag, followed by immunoprecipitation with anti-Flag antibodies and immunoblotting ith anti-p-Tyr antibodies. AAA mutant PDHA1 lacks PDK phosphorylation sites. C. Src inhibitors block tyrosine phosphorylation of exogenous PDHA1 in cancer cells. Indicated cancer cells were transduced with lentiviral PDHA1-Flag. Cells were treated with Src inhibitors PP2 (5 μM), SU (2 μM) or SA (2 μM), then harvested for immunoprecipitation with anti-Flag antibodies and immunoblotting for tyrosine phosphorylation. D. Endogenous PDHA1 proteins are tyrosine-phosphorylated in Src-activated cancer cells. Whole cell lysates were prepared from indicated cancer cells, and subjected to either immunoblotting with anti-PDHA1 antibodies or immunoprecipitation with anti-p-Tyr antibodies, followed by immunoblotting with anti-PDHA1. E. Src inhibitors abolish tyrosine phosphorylation of endogenous PDHA1. AsPC1 cancer cells were treated with PP2 (5 μM), SA (1 μM) or SU (1 μM), followed by immunoprecipitation with anti-p-Tyr antibodies and immunoblotting with anti-PDHA1 antibodies. F. Src is required for tyrosine phosphorylation of endogenous PDHA1. AsPC1 cells were infected with lentiviral vector (pLKO) or shRNA targeting Src (shSrc). Src depletion efficiency was verified by quantitative RT-PCR. Cells were lysed for immunoprecipitation with anti-p-Tyr antibodies, followed by immunoblotting with anti-PDHA1 antibodies. G. Src phosphorylates Y289 of PDHA1. WT and tyrosine mutant forms of PDHA1 (aa 281-356) were fused to GST. The purified fusion proteins were incubated with recombinant Src enzyme, followed by immunoblotting with anti-p-Tyr antibodies.
