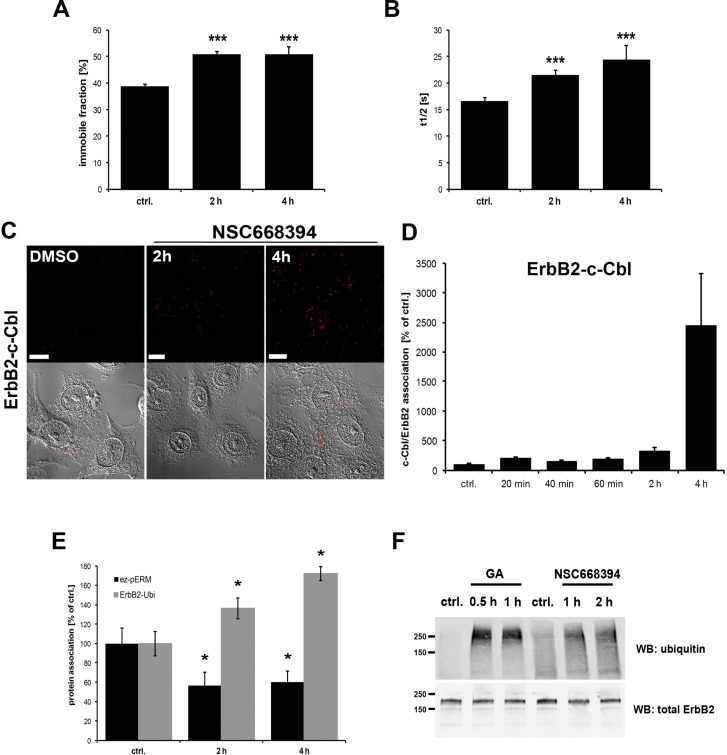
Figure 4. Effect of ERM inhibition on ErbB2 membrane mobility and association to ubiquitin.
(A, B) FRAP analysis of GFP-ErbB2 membrane mobility. SKBR3 cells were transfected for 16 h with GFP-ErbB2 plasmids and subsequently treated with NSC668394 for 2 h and 4 h. As described in the Methods part, specific regions were bleached and the recovery of the GFP-fluorescence was monitored and analyzed. Inhibition of ERM proteins by treatment with NSC668394 leads to a ~25% increase in the immobile ErbB2 fraction (A), accompanied by a significant increase of 20–30% in the t1/2 recovery time (B). (C) Representative confocal microscopy of ErbB2-c-Cbl PLA experiments and (D) quantification of ErbB2-c-Cbl PLA experiments. Cells were treated with DMSO alone or NSC668304 for the indicated periods of time and PLA experiments were subsequently performed in fixed and permeabilized cells. Inhibition of ERM proteins by NSC668394 strongly increased the association of ErbB2 with C-Cbl. Scale bars: 10 μm. (E) PLA experiments of ErbB2-ubiquitin association upon ERM inhibition. Cells were treated for 2 h and 4 h with NSC668394 and PLA experiments were performed. The phosphorylation status of ezrin was measured by using ezrin- and pERM- specific antibodies. Proximity of ErbB2 with ubiquitin was analyzed by the combination of ErbB2 and ubiquitin-specific antibodies. Reduction of pERM levels of 40% leads to 40–70% increase in ErbB2-ubiquitin association. All results are shown as mean +/− SEM (*P < 0.05; ***P < 0.001). (F) Immuoprecipitation of ErbB2-ubiquitin conjugates. Cells were treated with GA or NSC668394 or left untreated. Cell lysates were prepared and the input was probed for total ErbB2. Immunoprecipitations were performed with an anti-ErbB2 antibody. Treatment with GA as well as ERM inhibition significantly increases ubiquitination of ErbB2.