Figure 2. EBNA1 forms a complex with ERK2.
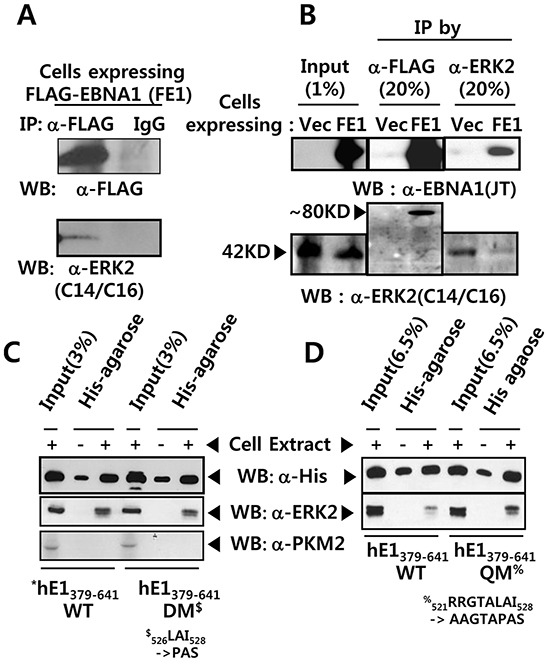
A. Anti FLAG M2 antibody pulled-down FLAG-tagged EBNA1 (FE1) and ERK2 in BJAB cell extract, while IgG control antibody did not. B. Reciprocal co-immunoprecipitation (co-IP) of FE1 and cellular ERK2. Stable BJAB cells expressing vector control (Vec) or FE1 were immunoprecipitated using anti-FLAG and anti-ERK2 antibodies. 1% input and 20% immune complex were resolved on 10% SDS protein gels and probed with anti-FLAG, anti-EBNA1, and anti-ERK2 antibodies. Despite low ERK2 IP efficiency in FE1 cells, substantial FE1 was co-immunoprecipitated by anti-ERK2 antibody. The low ERK2 IP efficiency in FE1 cells, compared to vector control, was due to experimental deviation seen in this specific case (See also Supplementary Figure S1). C–D. Pull-down assay using His-bind magnetic beads, followed by western blotting using anti-His tag and anti-ERK2 antibodies. Interaction with ERK2 was observed between hE1 a.a. 379-641 WT, DM, and quadruple mutant (QM) (521RRGTALAI528 → 521AAGTAPAS528). To rule out hE1 non-specific interaction with other cellular kinases, the same membrane was stripped and reprobed with anti-PKM2 antibody. Results show that mutations in the putative ERK docking motif of EBNA1 did not abrogate interaction between EBNA1 and ERK2.
