Figure 1. Structure Activity Relationship (SAR) of C188 and similar compounds.
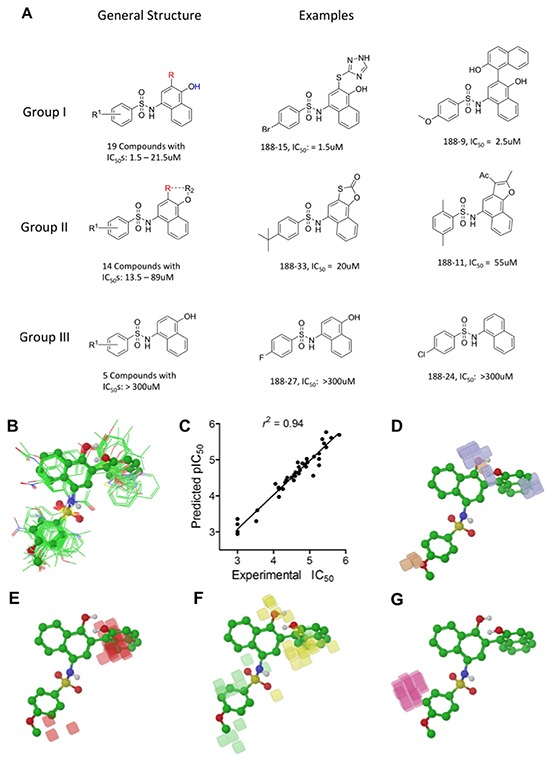
A. SAR grouping of 37 C188-like STAT3 probes. Thirty-seven of the 39 C188-like compounds can be divided into three structural groups with activity ranging from highest to lowest. The most potent Group I compounds contain a variety of groups, such as a triazole-3-yl-mercapto (188-15) or a hydroxynaphthalene (188-9), at the 3-position of the naphthylamine ring (the -R group highlighted in red). Group II compounds with intermediate potency contain a 5-membered ring that combines the 3-R and 4-OR2 groups, such as a furan (188-11). The least potent Group III probes do not contain a substitution at the 3-position. B–G. Quantitative SAR of compounds. Alignment of C188 and C188-1 through C188-39 showing (B) only heavy atoms and polar hydrogens displayed for clarity, with C188-9 in ball and stick model; C. Correlation between experimental and predicted pIC50 values; D. Phase H-bond donor fields, superimposed with the aligned structure of C188-9, blue favorable, orange disfavored; E. hydrophobic fields, red favorable; F. electron-withdrawing fields, yellow favorable, light green disfavored; G. negative ionic fields, pink favorable.
