Figure 4. IR induces RIG-I binding to endogenous double-stranded RNAs.
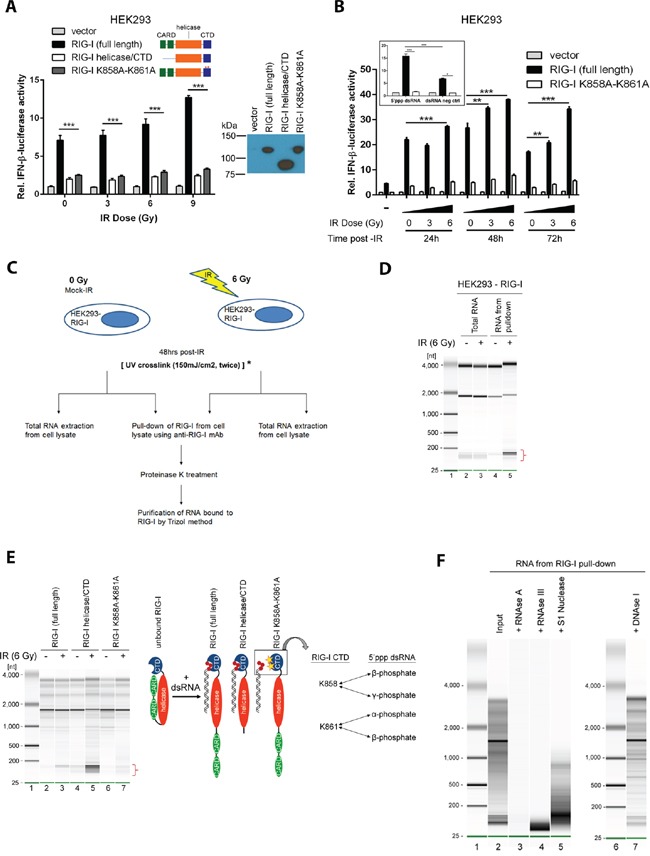
A. HEK293 reporter cells were irradiated after transfection with either an empty vector, a full length human RIG-I, a RIG-I lacking CARD domains (RIG-I helicase/CTD), or a RIG-I harboring K858A and K861A mutations in the C-terminal domain (RIG-I K858A-K861A), in addition to an IFN-beta promoter-driven luciferase construct. A Renilla reporter construct served as a transfection control. Data are presented as mean fold-change relative to the non-irradiated empty vector control. B. Donor HEK293 cells were either unirradiated or treated with IR (3 or 6 Gy). Total RNA was purified and transferred to independent batches of HEK293 reporter cells transfected by RIG-I constructs as described in (A). A synthetic double-stranded RNA construct comprised of 5′-triphosphorylated dsRNA and an unphosphorylated counterpart served as positive and negative controls, respectively (inset). C. Experimental design for isolation and purification of RNA bound to RIG-I after exposure to IR. *To validate RNA sequencing data by qPCR experiments, UV crosslinking was performed prior to cell lysis and immunoprecipitation of RIG-I. See methods for further details. D. Purified RNA from total cellular extracts (Lanes 2 and 3) and complexes with RIG-I (Lanes 4 and 5). Lane 1 is the marker. Data are representative of at least 3 independent experiments. E. HEK293 cells over-expressing the HA-tagged full length RIG-I (Lanes 2 and 3), the RIG-I helicase-CTD mutant (Lanes 4 and 5) and the RIG-I K858A-K861A CTD mutant (Lanes 6 and 7) were either un-irradiated or exposed to IR (6 Gy), lysed and incubated with anti-HA monoclonal antibody to pulldown the respective WT and mutant RIG-I proteins. RIG-I diagrams illustrate the mechanism of RIG-I activation (adapted from [57]). In the inactive/unbound conformation, the CARD domain of RIG-I is folded to block the helicase domain from RNA binding RNA, but allows the CTD to search for its ligand. Upon binding of the blunt end of a dsRNA molecule to the CTD, the CARD domain opens to allow the helicase domain to bind the remaining dsRNA molecule. Absence of the CARD domain in the helicase/CTD mutant enables higher affinity binding to dsRNA ligands as compared to the full length RIG-I. The lysine residues at amino acid positions 858 and 861 have previously demonstrated importance in latching onto the 5′-triphosphorylated end of viral dsRNA ligands. F. RNA bound to RIG-I after exposure to IR (6 Gy) was treated with: RNase A (lane 3), dsRNA-specific RNase III (lane 4), single-strand specific nuclease S1 (lane 5) and DNase I (lane 7). Lane 2 shows the input and lanes 1 and 6 display markers.
