Figure 3.
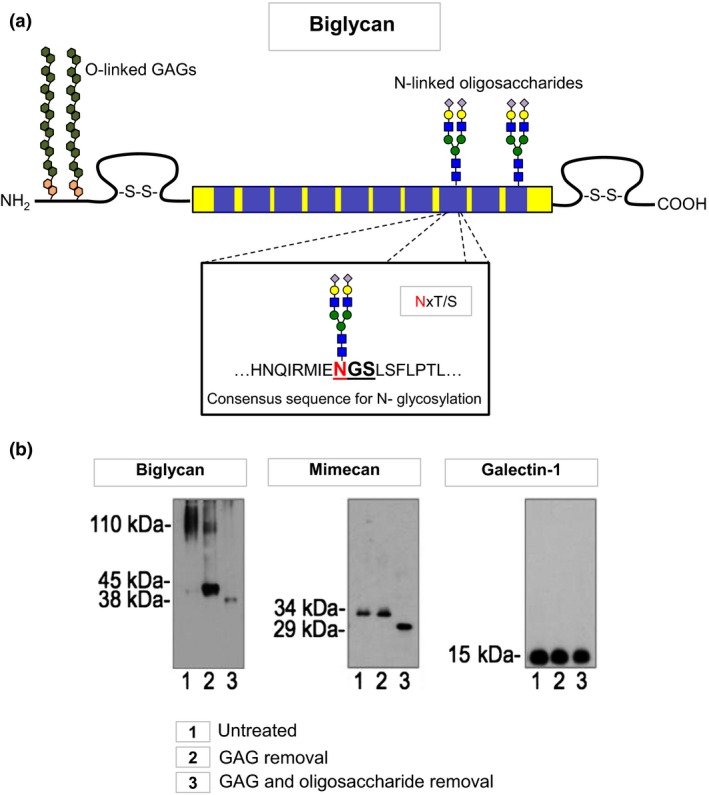
Glycosylation of extracellular matrix (ECM) proteins. More than 90% of ECM proteins are glycosylated, which can affect protein identification with antibodies. (a) Attachment of N‐linked oligosaccharides to ECM proteins occurs at specific consensus sequences. Biglycan is an example of a heavily glycosylated ECM protein. (b) Removal of glycosaminoglycans (GAGs) and small oligosaccharides or removal of GAGs alone affects protein migration by gel electrophoresis. Biglycan contains GAGs and oligosaccharides, and mimecan contains only N‐linked oligosaccharides, whilst galectin‐1 is a nonglycosylated ECM protein. The aim of glycoproteomics is the identification of the protein core and the attached glycan.
