Fig. 1.
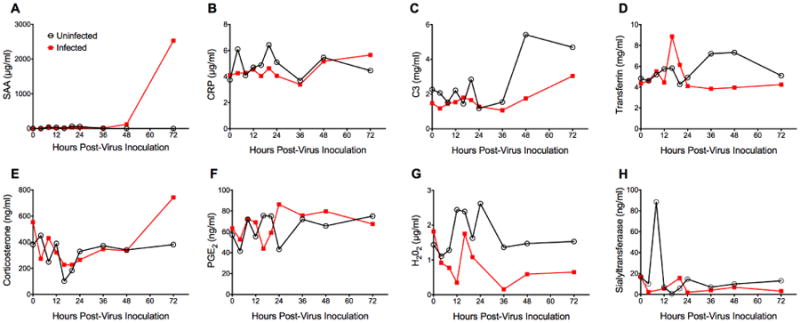
Screening of serological biomarker candidates for early detection of influenza virus disease in BALB/c mice. Mice (n=50) were intranasally infected with mouse-adapted influenza A/California/04/2009 (H1N1)pdm09 virus (1LD100). Control animals (uninfected) received dilution vehicle (MEM) only. At specific time points (0, 4, 8, 12, 16, 20, 24, 36, 48, 72 hours post-virus inoculation), 5 animals per cohort were sacrificed for serum collection. Survival and body weight were monitored daily (n=5, data not shown). The experiment was terminated 3 days post-virus inoculation. Serum was analyzed for levels of (A) Serum amyloid A (SAA), (B) C-reactive protein (CRP), (C) Complement factor 3 (C3), (D) Transferrin, (E) Corticosterone, (F) Prostaglandin E2 (PGE2), (G) H2O2, and (H) alpha-2,6-Sialyltransferase. SAA levels were quantified in three independent ELISA assays and CRP levels were determined in two independent ELISA assays. All other candidates were tested only once. Mean and SD values could not be calculated as serum samples were pooled per cohort to assure sufficient testing material for all markers. SAA showed the greatest potential as a biomarker candidate.
