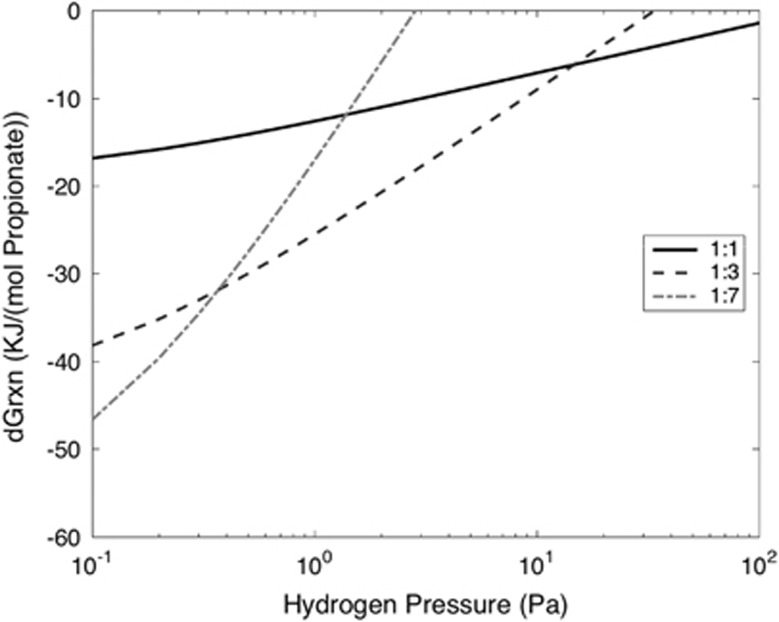
Figure 4.
Thermodynamic energy of three propionate degrading reactions (from Table 1), normalised per propionate, at different ambient H2 pressures. Each reaction produces H2 as a byproduct, and the line type indicates the reaction stoichiometry between the propionate and hydrogen (as shown in the legend). The conditions used to calculate the reaction thermodynamics are pH=7, 1 mmol l−1 of ambient propionate, acetate and HCO3− and varying ambient H2 pressures as shown on the x axis.