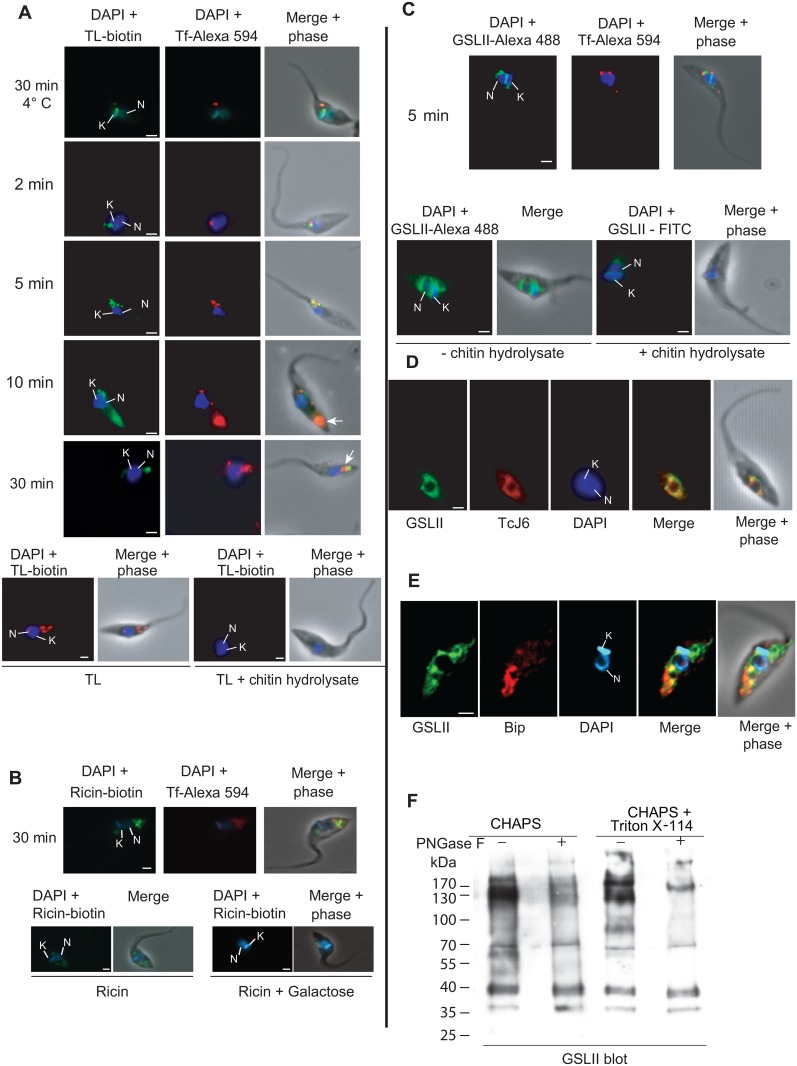
Fig 2. Localization of TL and GSLII binding sites in T. cruzi.
Endocytosis kinetics of fluorescent Alexa Fluor 594 conjugated Tf was performed in order to follow T. cruzi endocytic pathway from the flagellar pocket/cytostome to the reservosomes. Parasites were fixed at different time points and probed with biotinylated TL (A), biotinylated ricin (B) or Alexa 488 conjugated GSLII (C). The addition of chitin hydrolysate clearly shows inhibition of TL and GSLII staining. (A) Co-localization of biotinylated-TL (green) and Tf (red). (B) Co-localization of biotinylated-ricin (green) and Tf (red). Addition of 200 mM galactose abolished the ricin staining. (C) Co-localization of Alexa 488 conjugated GSLII (green) and Tf (red). (D) Co-localization of Alexa 488 conjugated GSLII (green) and TcJ6 (red). (E) Co-localization of Alexa 488 conjugated GSLII (green) and anti-BiP (red). (F) GSLII blotting of cell extracts enriched by GSLII chromatography. GSLII blots of T. cruzi CHAPS- and Triton-soluble (CHAPS+Triton X-114) cell lysate fractions were enriched by GSLII chromatography and then treated (+) or not (-) with PNGase F. Blots were probed with biotinylated-GSLII. The GSLII blot indicates the presence of N-acetylglucosamine modification in both soluble and membrane fractions. Treatment of the fractions with PNGase F decreased the reactivity of GSLII confirming N-glycoprotein type modification.