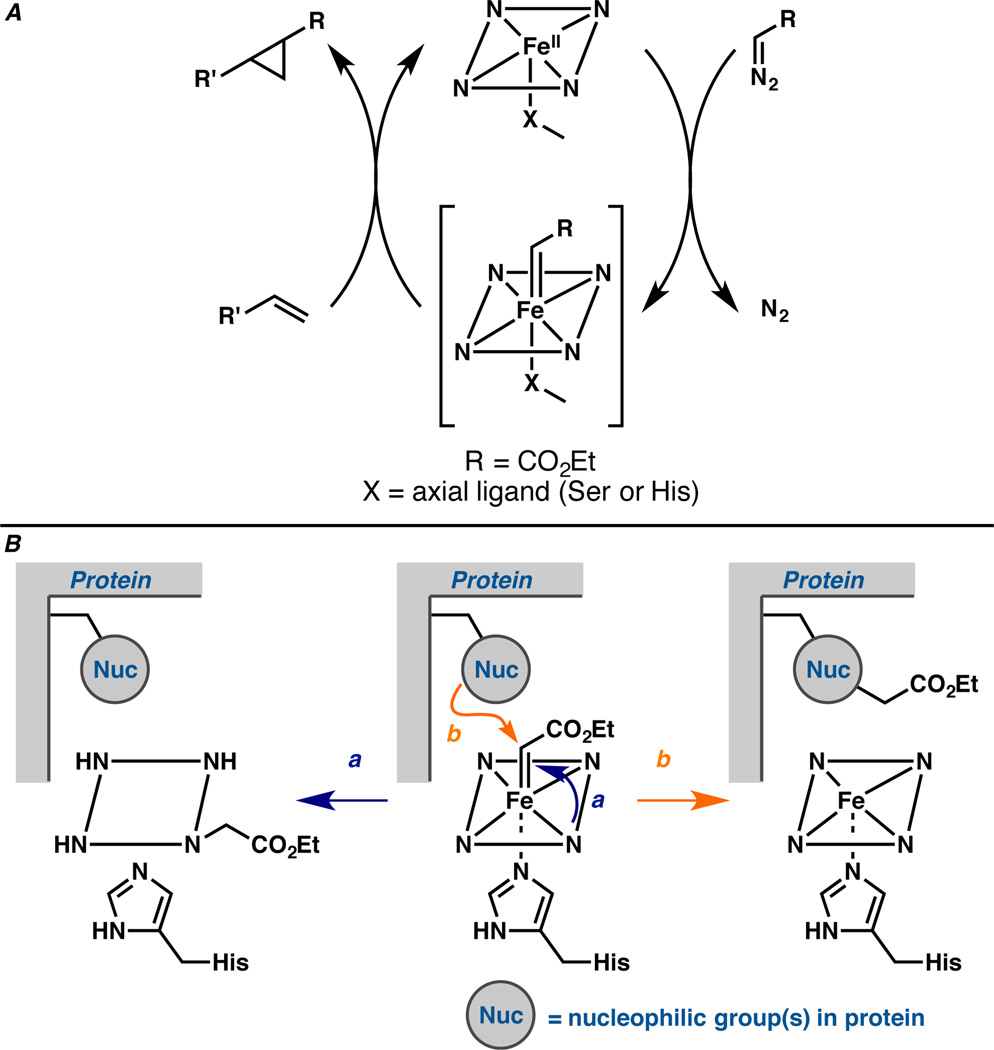
Figure 1.
A. Proposed catalytic cycle for heme protein-catalyzed carbene transfer to an olefin starts from the reduced ferrous state, which undergoes reaction with a diazo compound to form the putative iron carbenoid. The carbenoid reacts with an olefin, forming the cyclopropane product and regenerating the ferrous heme protein. B. Proposed mechanism-based inactivation via (a) porphyrin and (b) side chain modification involving the same iron carbenoid intermediate that mediates productive olefin cyclopropanation.