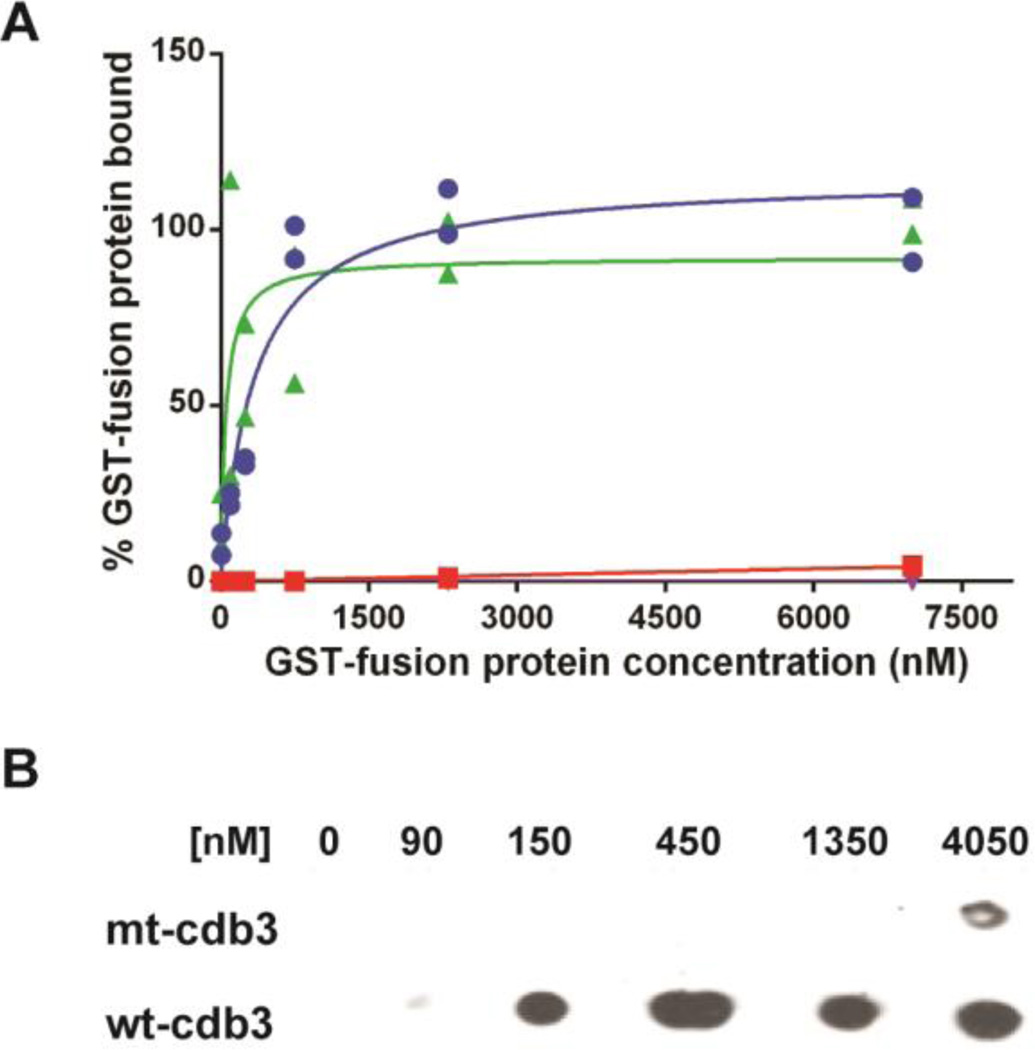
Figure 3.
Binding of β-adducin tail and ankyrin to wild-type and mutant cdb3. Increasing concentrations of GST-β-adducin tail or GST-D3D4-ankyrin were incubated with immobilized wild-type or mutated cdb3 in which glutamates 252 and 254 were mutated to lysines. Beads were washed with PBS and eluted with 250 mM imidazole, and GST activity was quantified as a measure of bound adducin or ankyrin. (A) Binding of β-adducin tail domain to wild type cdb3 (blue, Kd = 260 nM), mutated cdb3 (red), or BSA (purple) is shown as a function of GST-β-adducin tail domain concentration. Binding of D3D4-ankyrin (green) to mutated cdb3 is shown as a function of GST-D3D4-ankyrin concentration. Individual data points (n=2) are plotted with estimated binding curves. (B) Dot blot analysis of β-adducin tail binding to purified wild-type and mutant cdb3. Wild-type or mutated band 3 was immobilized on nickel beads and incubated with increasing concentrations of GST-β-adducin tail domain. Beads were pelleted, washed 4×, and eluted with 250 mM imidazole in PBS. Eluted proteins were transferred to a nitrocellulose membranes and probed for GST-β-adducin tail with an anti-GST polyclonal antibody.