Figure 17.
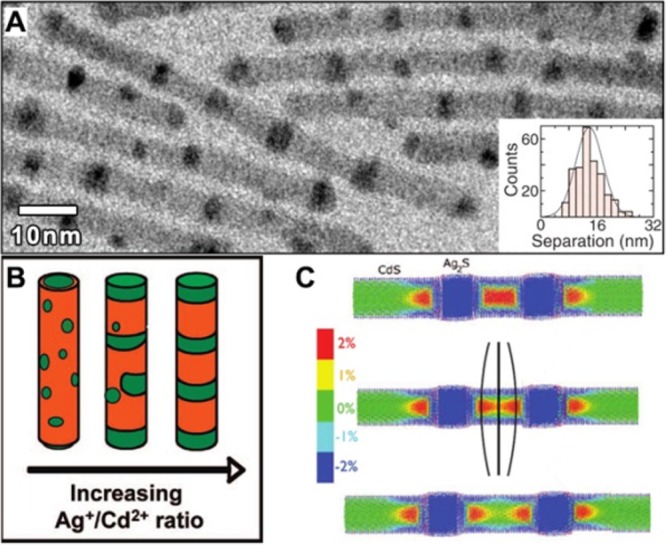
(A) TEM image of Ag2S-CdS superlattices formed through partial CE. (Inset) Histogram of Ag2S segment spacing (center-to-center). The average spacing is 13.8 ± 3.8 nm. Adapted with permission from ref (71). Copyright 2007 The American Association for the Advancement of Science. (b) Sketch representing the development of the morphology of binary nanorods produced by CE at increasing amounts of Ag+ added to CdS NRs. Reproduced from [Sadtler, B.; Demchenko, D. O.; Zheng, H.; Hughes, S. M.; Merkle, M. G.; Dahmen, U.; Wang, L.-W.; Alivisatos, A. P. Selective facet reactivity during cation exchange in cadmium sulfide nanorods J. Am. Chem. Soc. 2009, 131, 5285–5293]. Copyright 2009 American Chemical Society. (C) Color maps of the z-component of strain in the interatomic bonds, for a 4.8 nm diameter Ag2S/CdS striped NR. The segment separations are 6, 8, and 10 nm. The color map limits indicate a strain ranging from −2% (compression, blue) to 2% (tension, red). The atoms are pulled in opposite directions (indicated by the black lines at the center of the second rod), which leads to an increased interaction energy when the segments separation decreases. Reproduced from [Demchenko, D. O.; Robinson, R. D.; Sadtler, B.; Erdonmez, C. K.; Alivisatos, A. P.; Wang, L.-W. Formation Mechanism and Properties of CdS-Ag2S Nanorod Superlattices ACS Nano2008, 2, 627–636]. Copyright 2008 American Chemical Society.
