FIG 2.
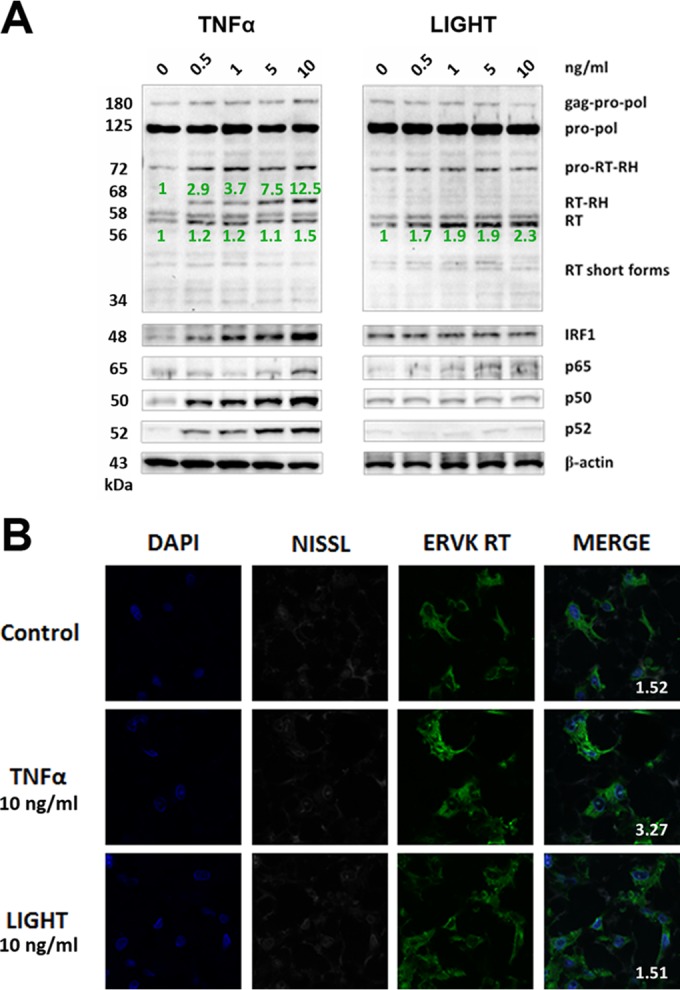
TNF-α, but not LIGHT, markedly enhances ERVK polyprotein and RT expression in neurons. ReNcell CX cell-derived neurons were treated with various doses of TNF-α or LIGHT for 24 h. Western blotting and confocal microscopy were used to detect alterations in ERVK RT, IRF1, and NF-κB p65, p50, and p52 protein levels. (A) Western blot (cropped) showing that TNF-α strongly induced ERVK polyprotein (180 and 125 kDa) expression and cleavage to produce the small, 56/58-kDa RT without RNase H and the larger, 68-kDa RT with RNase H, concomitantly with upregulation of IRF1 and NF-κB protein levels. In comparison, LIGHT enhanced the expression of the RT subunit without RNase H but not RT with the RNase H domain, suggesting that optimal RT activity may occur during exposure of neurons to TNF-α. β-Actin was used as the loading control (n = 3). Quantification of the 56-kDa and 68-kDa bands is depicted in green. (B) Representative confocal micrographs depicting TNF-α-mediated induction of ERVK RT expression in neurons. The fluorescent Nissl stain was used to identify neurons (n = 3). Quantification of the ERVK RT/DAPI staining ratio is presented in each merged micrograph.
