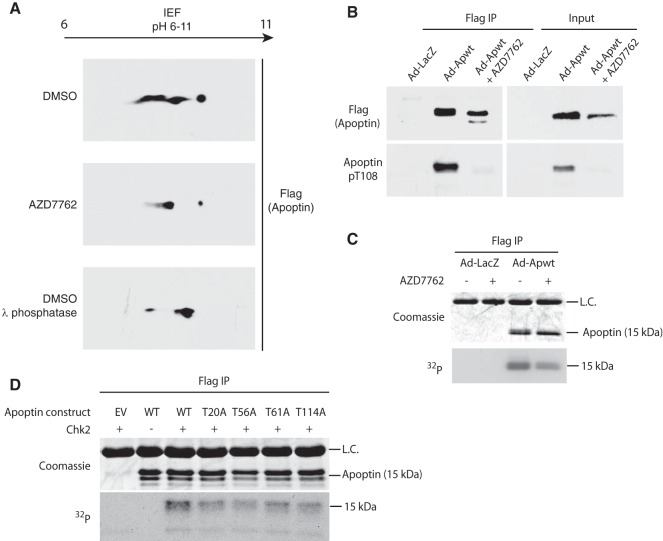
FIG 5.
Apoptin is a checkpoint kinase substrate. (A) H1299 cells were infected with Ad-Apwt and treated with DMSO or 6.3 μM AZD7762 (Chk-i). At 24 h postinfection, Flag-apoptin was purified by immunoprecipitation under stringent conditions and resolved in the first dimension with a pH 6 to 11 gradient and then analyzed by SDS-PAGE in the second dimension. Immunoblotting for Flag was performed to visualize Flag-apoptin peptides. Lambda-phosphatase treatment of purified Flag-apoptin served as a positive control. IEF, isoelectric focusing. (B) H1299 cells were treated with DMSO or 6.3 μM Chk-i and infected with Ad-Apwt or Ad-LacZ for 24 h. Whole-cell extracts were prepared for immunoprecipitation (IP) of Flag complexes and immunoblot analysis with the indicated antibodies (pT108, phospho-specific antibody [Ab] for phosphorylated Thr108). (C) H1299 cells were infected with Ad-Apwt or Ad-LacZ and treated with DMSO or 6.3 μM AZD7762. At 23 h postinfection, the medium was changed to include 250 mCi/ml [32P]orthophosphate. Following 1 h of labeling, Flag-apoptin was purified by immunoprecipitation under stringent conditions and analyzed by SDS-PAGE. (D) H1299 cells were transfected with the indicated constructs. At 48 h posttransfection, Flag-apoptin was immunoprecipitated with anti-Flag resin. The resin was then incubated with 100 ng Chk2 and [γ-32P]ATP for 15 min at 30°C. Residual γ-ATP was washed out, and 4× sample buffer was added to the samples, which were then analyzed by SDS-PAGE. L.C. indicates IgG light chain.