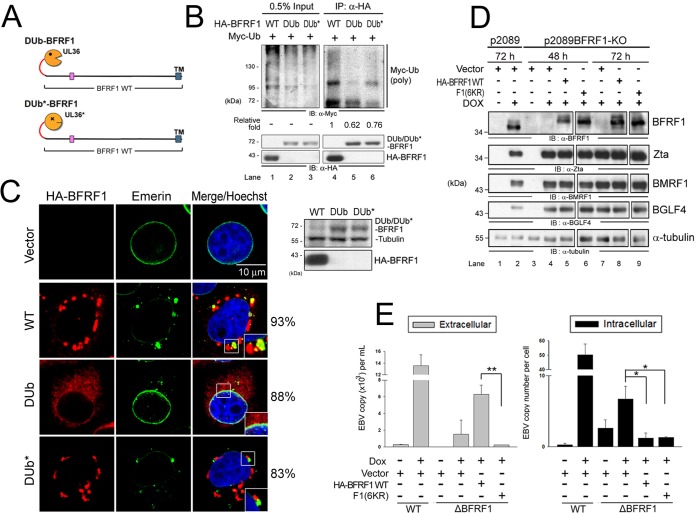
FIG 5.
Ubiquitination is required for BFRF1 function in vesicle induction and virus replication. (A) Schematic representation of fusion of the DUb of HSV-1 UL36 and its inactive form (DUb*) onto BFRF1. (B) Lysates of HeLa cells with expression of HA-BFRF1 WT, HA-DUb-BFRF1, or HA-DUb*-BFRF1 and Myc-Ub were harvested and immunoprecipitated with antibody against HA. The immunocomplexes were then detected by antibodies against Myc and HA by immunoblotting. (C) Confocal images of HA-BFRF1- and DUb- or DUb*-HA-BFRF1-transfected HeLa cells immunostained for HA (red) and emerin (green). The boxed regions are enlarged in the insets. The protein expression of BFRF1 WT, DUb-BFRF1, and DUb*-BFRF1 was also confirmed by immunoblotting using HA antibody. Tubulin served as a protein-loading control. The percentages of cells showing the representative pattern are indicated. (D) EBV-positive 293TetEZ/p2089 and 293TetEZ/p2089BFRF1-KO cells transfected with HA-BFRF1 WT or F1(6KR) or vector were mock treated or treated with DOX to induce Rta expression and harvested at 72 h postinduction. Viral protein expression was detected by immunoblotting with antibodies against BFRF1 (E10), Zta (4F10), BMRF1 (88A9), and BGLF4 (2616). α-Tubulin served as a protein-loading control. (E) Viral DNAs in the culture supernatant (extracellular) and transfected cells (intracellular) were harvested simultaneously and subjected to qPCR analysis targeting the EBV DNA BamHI W fragment. Statistically significant differences between HA-BFRF1 WT and F1(6KR) expression levels in supernatant and transfected cells are indicated by the asterisks. *, P < 0.05; **, P < 0.01.