Abstract
Background:
We report on a patient who appeared to show recovery of an injured anterior cingulum via an aberrant neural tract between an injured cingulum and the basalis nucleus of Meynert following traumatic brain injury (TBI), which was demonstrated on diffusion tensor tractography (DTT).
Methods:
A 47-year-old male who had suffered a pedestrian traffic accident underwent conservative management for diffuse traumatic axonal injury. When starting rehabilitation at 6 weeks after onset, evaluation using the Mini-Mental State Examination (MMSE) could not be performed due to the severity of his cognitive dysfunction. The patient showed improvement of cognitive dysfunction on MMSE with 10 at 2 months, 13 at 6 months, and 26 at 10 months after onset.
Results:
On 6-week DTT, discontinuation superior to the genu of the corpus callosum was observed in both cingulums. However, on 6-month DTT, the discontinued anterior part of the right cingulum was elongated anteriorly, not through the cingulum, but through the anterolateral subcortical white matter of the cingulum, while on 10-month DTT, this elongated neural tract of the right cingulum was connected to the right basalis nucleus of Meynert in the basal forebrain.
Conclusion:
Recovery of an injured anterior cingulum via an aberrant neural tract between an injured cingulum and Ch 4 was demonstrated in a patient with TBI. Our result appears to suggest a mechanism for recovery of an injured cingulum following brain injury.
Keywords: aberrant neural tract, cingulum, cognition, diffusion tensor tractography, traumatic axonal injury
1. Introduction
The cingulum is an important neural tract involved in various functions for cognition, including emotion, memory, attention, motivation, and learning.[1] The cingulum, which originates from the nucleus basalis of Meynert (Ch 4), obtains cholinergic innervation from two other cholinergic nuclei (the medial septal nucleus [Ch1], and the vertical nucleus of the diagonal band [Ch2]) and contains the medial cholinergic pathway; therefore, injury of the cingulum can lead to cognitive impairment, particularly memory impairment.[2,3]
Diffusion tensor tractography (DTT), derived from diffusion tensor imaging (DTI), enables 3D visualization and estimation of the cingulum.[4] Many studies using DTT have reported on injury of the cingulum in patients with brain injury,[5–7] however little is known about the mechanism for recovery of an injured cingulum.[8–12]
In the present study, we attempted to demonstrate the recovery of an injured cingulum via an aberrant neural tract in a patient with traumatic brain injury (TBI).
2. Case report
A 47-year-old, right-handed male who had suffered a pedestrian traffic accident underwent conservative management for diffuse traumatic axonal injury at the department of neurosurgery of a university hospital. The patient lost consciousness for 3 days from the time of TBI and the Glasgow Coma Scale score was 11 when he arrived at the hospital.[13] He was transferred to the department of rehabilitation of the same university hospital for rehabilitation at 6 weeks after onset. A subdural hygroma located in the left frontal convexity was observed on brain magnetic resonance imaging at 6 weeks after onset (Fig. 1A). Evaluation using the Mini-Mental State Examination (MMSE) and the Wechsler adult intelligence scale could not be performed due to the severity of his cognitive dysfunction.[14,15] The patient underwent comprehensive rehabilitation, including cognitive therapy, until 10 months after onset. He showed improvement of cognitive dysfunction on the MMSE with 10 (full score: 30) at 2 months, 13 at 6 months, and 26 at 10 months after onset. Total IQ was 90 on the Wechsler adult intelligence scale at 10 months after onset. The patient provided signed, informed consent, and the study protocol was approved by our institutional review board.
Figure 1.
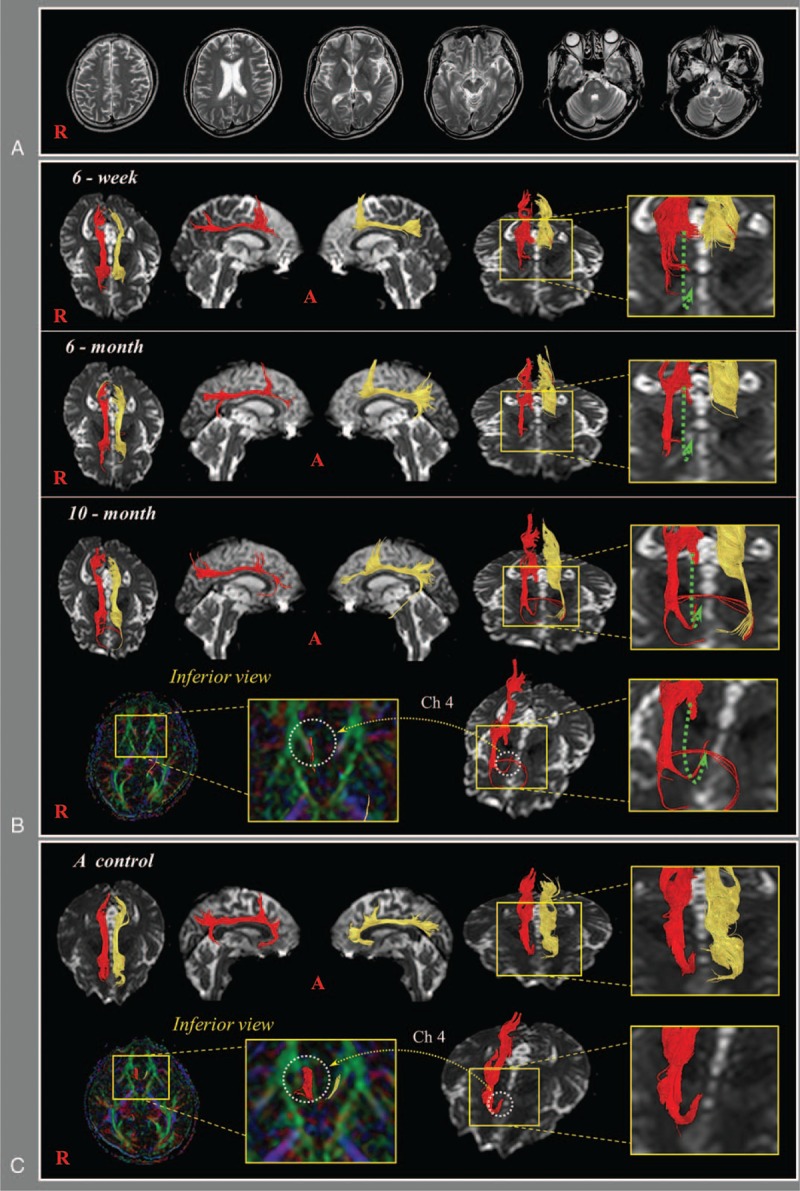
(A) Brain magnetic resonance imaging at 6 weeks after onset showed a subdural hygroma located in the left frontal convexity. (B) DTTs of the patient; 6-week DTT: discontinuation (green arrows) superior to the genu of the corpus callosum is observed in both cingulums, 6-month DTT: the discontinued anterior part of the right cingulum is elongated anteriorly through the anterolateral subcortical white matter of the cingulum (blue arrow), 10-month DTT: this elongated neural tract of the right cingulum is connected to the right nucleus basalis of Meynert (Ch 4) in the basal forebrain. (C) DTT images of the cingulum in a normal control subject (48-year-old male). DTT = diffusion tensor tractography.
DTI data were acquired three times (6 weeks, 6 months, and 10 months after onset) using a 1.5 T with a 6-channel head coil. The imaging parameters were as follows: field of view = 240 mm × 240 mm; reconstructed to matrix = 192 × 192; acquisition matrix = 96 × 96; b = 1000 s/mm2; NEX = 1; TR = 10,398 ms; TE = 72 ms; EPI factor = 59; slice gap = 0 mm; a slice thickness of 2.5 mm; and parallel imaging reduction factor (SENSE factor) = 2. Using the fiber assignment continuous tracking algorithm, fiber tracking was performed within the DTI task card software (Philips Extended MR WorkSpace 2.6.3).[16] For analysis of the cingulum, the first ROI was placed on the most posterior coronal slice, where the genu of the corpus callosum was seen clearly in full profile (green color). The second ROI was placed on the middle coronal slice, where the body of the corpus callosum was seen in full profile (green color). Termination criteria were fractional anisotropy less than 0.15 and an angle change of more than 27°.[17]
On 6-week DTT, discontinuation superior to the genu of the corpus callosum was observed in both cingulums. However, on 6-month DTT, the discontinued anterior part of the right cingulum was elongated anteriorly, not through the cingulum but through the anterolateral subcortical white matter of the cingulum. On 10-month DTT, this elongated neural tract of the right cingulum was connected to the right Ch 4 in the basal forebrain and the discontinued anterior part of the left cingulum was elongated anteriorly through the anterolateral subcortical white matter of the cingulum (Fig. 1B). Results of DTT were compared with a normal subject (48-year-old male) (Fig. 1C).
3. Discussion
In this study, changes of injured cingulums observed on DTTs with changes of cognitive impairment were followed up in a patient with TBI. Discontinuation of both cingulums above the genu of the corpus callosum was observed on 6-week DTT. Subsequently, on 6-month DTT, the discontinued right cingulum was elongated anteriorly through the anterolateral subcortical white matter of the cingulum. On 10-month DTT the elongated anterior cingulum was finally connected to the right Ch4 in the basal forebrain, 1 of 4 cholinergic nuclei in the basal forebrain.[2,3] As a result, it appeared that the right Ch4 and the discontinued right anterior cingulum were connected, not through the cingulum, but through the aberrant neural tract which passed through the anterolateral subcortical white matter of the cingulum. These changes of the right cingulum observed on DTT appeared to indicate that the injured cingulum had recovered to obtain cholinergic innervation from Ch4, which coincided with the improvement of cognitive impairment in terms of MMSE (6 weeks: uncheckable, 6 months: 13, and 10 months: 26).[18] No specific lesion was observed in the basal forebrain and cingulum areas, thus it appeared that traumatic axonal injury in the anterior portion of both cingulums was the result of the traffic accident.[19]
Since the introduction of DTI, 3 mechanisms for recovery of injured anterior cingulums in order to obtain cholinergic innervation from cholinergic nuclei have been reported in patients with brain injury: first, recovery of the injured anterior cingulum through the normal cingulum pathway; second, recovery of the injured anterior cingulum through the crossed neural connection to the opposite cingulum; and third, recovery via the neural tract between the injured cingulum and brainstem cholinergic nuclei.[8–12] To the best of our knowledge, this is the first study to demonstrate the recovery of an injured cingulum via an aberrant neural tract between the injured anterior cingulum and Ch 4. However, the limitation of DTT should be considered: crossing or kissing fibers and partial volume effects could cause false positive results throughout the white matter of the brain.[20]
In conclusion, recovery of an injured anterior cingulum via an aberrant neural tract between an injured cingulum and Ch4 was demonstrated in a patient with TBI. The results of this study appear to suggest a mechanism for recovery of an injured cingulum following brain injury. However, because it is a case report, this study is limited; therefore, conduct of further complementary studies including larger numbers of patients is warranted.
Acknowledgments
This work was supported by the Medical Research Center Program (2015R1A5A2009124) through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning.
Footnotes
Abbreviations: DTI = diffusion tensor imaging; DTT = diffusion tensor tractography; TBI = traumatic brain injury.
The authors have no conflicts of interest to declare.
References
- 1.Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb Cortex 1992; 2:435–443. [DOI] [PubMed] [Google Scholar]
- 2.Selden NR, Gitelman DR, Salamon-Murayama N, et al. Trajectories of cholinergic pathways within the cerebral hemispheres of the human brain. Brain 1998; 121 (pt 12):2249–2257. [DOI] [PubMed] [Google Scholar]
- 3.Lucas-Meunier E, Fossier P, Baux G, et al. Cholinergic modulation of the cortical neuronal network. Pflugers Arch 2003; 446:17–29. [DOI] [PubMed] [Google Scholar]
- 4.Concha L, Gross DW, Beaulieu C. Diffusion tensor tractography of the limbic system. AJNR Am J Neuroradiol 2005; 26:2267–2274. [PMC free article] [PubMed] [Google Scholar]
- 5.Hong JH, Jang SH, Kim OL, et al. Neuronal loss in the medial cholinergic pathway from the nucleus basalis of Meynert in patients with traumatic axonal injury: a preliminary diffusion tensor imaging study. J Head Trauma Rehabil 2012; 27:172–176. [DOI] [PubMed] [Google Scholar]
- 6.Jang SH, Kim SH, Kim OR, et al. Cingulum injury in patients with diffuse axonal injury: a diffusion tensor imaging study. Neurosci Lett 2013; 543:47–51. [DOI] [PubMed] [Google Scholar]
- 7.Abdul-Rahman MF, Qiu A, Sim K. Regionally specific white matter disruptions of fornix and cingulum in schizophrenia. PLoS One 2011; 6:e18652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeo SS, Chang MC, Kim SH, et al. Neural connection between injured cingulum and pedunculopontine nucleus in a patient with traumatic brain injury. NeuroRehabilitation 2012; 31:143–146. [DOI] [PubMed] [Google Scholar]
- 9.Seo JP, Jang SH. Recovery of injured cingulum in a patient with brain injury: diffusion tensor tractography study. NeuroRehabilitation 2013; 33:257–261. [DOI] [PubMed] [Google Scholar]
- 10.Yoo JS, Kim OL, Kim SH, et al. Relation between cognition and neural connection from injured cingulum to brainstem cholinergic nuclei in chronic patients with traumatic brain injury. Brain Inj 2014; 28:1257–1261. [DOI] [PubMed] [Google Scholar]
- 11.Seo JP, Jang SH. Unusual neural connection between injured cingulum and brainstem in a patient with subarachnoid hemorrhage. Neural Regen Res 2014; 9:498–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwon HG, Jang SH. Recovery of injured cingulum in a patient with traumatic brain injury. Neural Regen Res 2015; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974; 2:81–84. [DOI] [PubMed] [Google Scholar]
- 14.Wechsler D. WAIS-R, Wechsler Adult Intelligence Scale- Revised, Manual: Psychological Corporation, 1981. [Google Scholar]
- 15.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198. [DOI] [PubMed] [Google Scholar]
- 16.Mori S, Crain BJ, Chacko VP, et al. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol 1999; 45:265–269. [DOI] [PubMed] [Google Scholar]
- 17.Hong JH, Choi BY, Chang CH, et al. Injuries of the cingulum and fornix after rupture of an anterior communicating artery aneurysm: a diffusion tensor tractography study. Neurosurgery 2012; 70:819–823. [DOI] [PubMed] [Google Scholar]
- 18.Wolk DA, Budson AE. Memory systems. Continuum (Minneap Minn) 2010; 16:15–28. [DOI] [PubMed] [Google Scholar]
- 19.Povlishock JT, Christman CW. The pathobiology of traumatically induced axonal injury in animals and humans: a review of current thoughts. J Neurotrauma 1995; 12:555–564. [DOI] [PubMed] [Google Scholar]
- 20.Yamada K, Sakai K, Akazawa K, et al. MR tractography: a review of its clinical applications. Magn Reson Med Sci 2009; 8:165–174. [DOI] [PubMed] [Google Scholar]


