Figure 4.
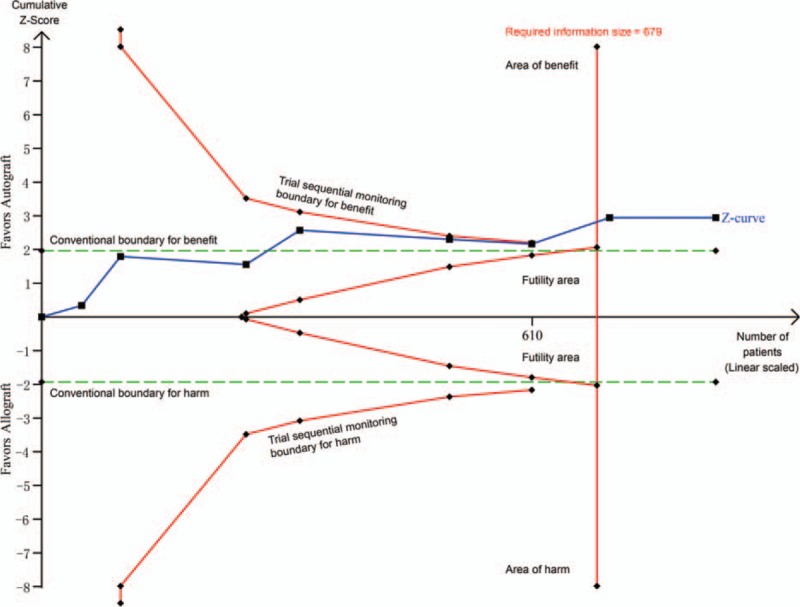
Trial sequential analysis of 8 trials comparing autograft with allograft for clinical failure. Trial sequential analysis of 8 trials (black square fill icons) illustrating that the cumulative z-curve crossed both the traditional boundary and the trial sequential monitoring boundary and the required information size had been reached, suggesting further trials were not necessary and the inferences would not be changed. A diversity adjusted required information size of 679 patients was calculated using α = 0.05 (2 sided), β = 0.20 (power 80%), a RR reduction of 49.76% based on trials with adequate allocation concealment, and an event proportion of 12.70% in the control arm. X-axis: the number of patients randomized; Y-axis: the cumulative Z-score; Horizontal green dotted lines: conventional boundaries (upper for benefit, Z-score = 1.96, lower for harm, Z-score = −1.96, 2-sided P = 0.05); Sloping red full lines with black square fill icons: trial sequential monitoring boundaries calculated accordingly; Blue full line with black square fill icons: Z-curve; Vertical red full line: required information size calculated accordingly.
