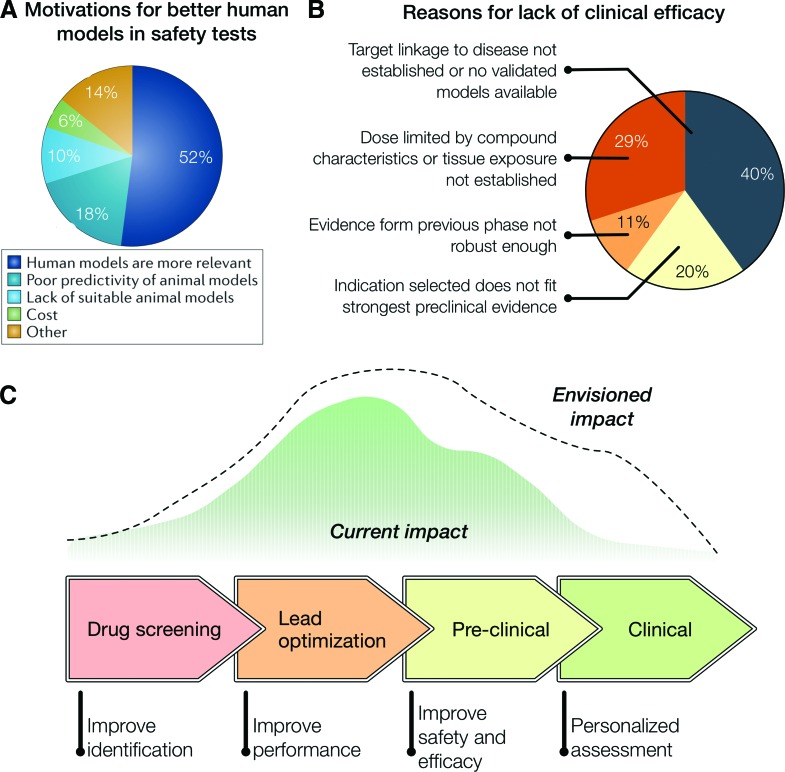
FIG. 2.
(A) Survey on the motivations behind adopting human tissue-based approaches for safety pharmacology studies.31 (B) AstraZeneca's small-molecule projects from 2005 to 2010. the terminated projects were analyzed to understand the causes for failure and identify potential predictors of success (total of 28 projects).8 (C) Impact of organs-on-a-chip technologies on the drug development pipeline. [(A) adapted from Holmes et al.31; (B) adapted from Cook et al.8]. Color images available online at www.liebertpub.com/aivt