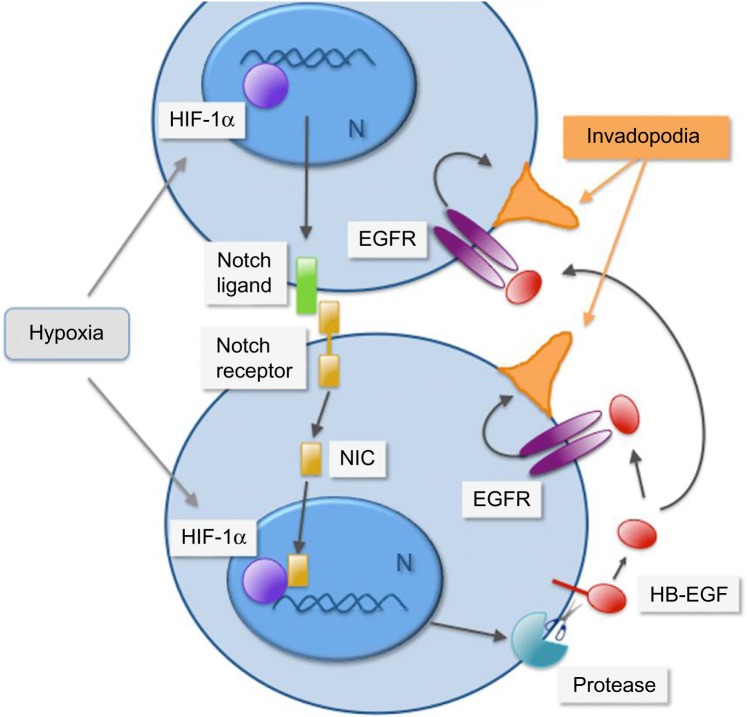
Figure 5.
Cell-contact-dependent signaling and paracrine activation of the EGF receptor are coupled under hypoxia to promote invadopodia formation in pancreatic cancer cells.
Notes: In pancreatic cancer cells, hypoxia promotes HIF-1α-dependent activation of the Notch signaling pathway, which mediates cell-contact-dependent signaling upon binding of a transmembrane ligand to a transmembrane receptor expressed in adjacent cells. Upon ligand binding, the receptor is cleaved by proteolysis to release a Notch intracellular domain (NIC) that travels to the nucleus (N) and partners with HIF-1α to promote gene transcription. Activation of Notch signaling under hypoxia promotes protease-dependent shedding of the EGF receptor ligand HB-EGF (red). Upon shedding, the transmembrane HB-EGF becomes soluble and signals in autocrine, juxtacrine, or paracrine fashions. Activation of the EGF receptor by soluble HB-EGF promotes invadopodia (depicted in orange) formation and increases cancer cell invasiveness. Adapted with permission from Díaz B, Yuen A, Iizuka S, Higashiyama S, Courtneidge SA. Notch increases the shedding of HB-EGF by ADAM12 to potentiate invadopodia formation in hypoxia. J Cell Biol. 2013;201(2):279–292.125
Abbreviations: EGF, epidermal growth factor; EGFR, epidermal growth factor receptor; HB-EGF, heparin-binding epidermal growth factor; HIF-1α, hypoxia-inducible factor 1α.