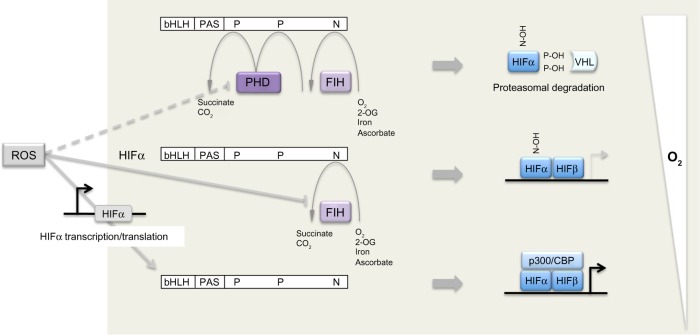
Figure 1.
Dual regulation of hypoxia-inducible factor (HIF)-α by oxygen-dependent prolyl and asparaginyl hydroxylation.
Notes: In the presence of oxygen, prolyl hydroxylase domain (PHD) enzymes and factor inhibiting HIF (FIH) hydroxylate two proline (P) and one asparaginyl (N) residues, respectively. Hydroxylated proline residues (P-OH) target HIF-α for proteasomal degradation by enabling recognition by the von Hippel-Lindau tumor suppressor protein (pVHL) product, an ubiquitin E3 ligase. Proline hydroxylation is inhibited in the presence of modest hypoxia, allowing HIF-α to escape proteolytic destruction and form a transcription factor in complex with HIF-β. More severe hypoxia results in the inhibition of hydroxylation of the asparaginyl residue (N-OH), allowing for the recruitment of the transcriptional co-activators p300/CREB (cAMP-response-element-binding protein) binding protein to enhance HIF-mediated gene transcription. Reactive oxygen species (ROS) can act at multiple points to modulate the HIF hydroxylase pathway: for example, by inhibiting FIH (and to a lesser extent, PHD enzymes) as well as by enhancing HIF-α transcription and translation.
Abbreviations: PAS, PER-AHR/ARNT-SIM; PER, period circadian protein; AHR, aryl-hydrocarbon receptor; ARNT, aryl-hydrocarbon-receptor nuclear translocator; SIM, single-minded protein; bHLH, basic-helix-loop-helix; 2-OG, 2-oxoglutarate.