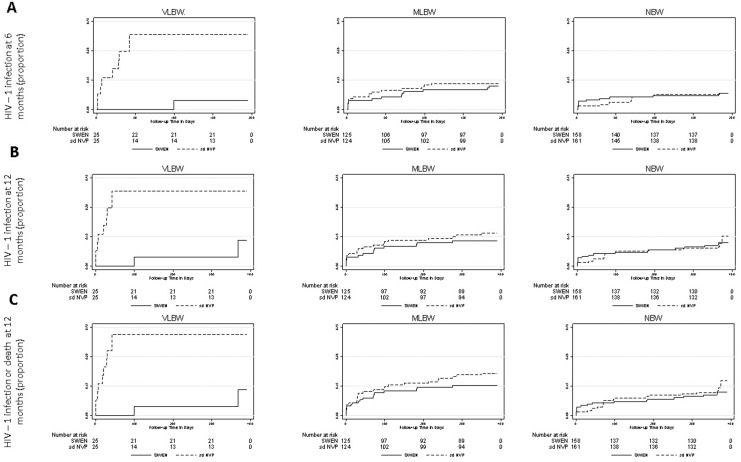
Fig 1. Kaplan-Meier plots of risk of HIV-1 infection (A and B) and HIV-1 infection or death (C) by treatment arm stratified by infant birth weight group.
(A) HIV-1 infection by 6 months. (B) HIV-1 infection by 12 months. (C) HIV-1 infection or death by 12 months. Estimated risk of each efficacy outcome is shown for infants randomized to six-week extended-dose nevirapine (SWEN) and single-dose nevirapine (SD) as a solid line and dashed line, respectively, within each infant birth weight group. Birth weight groups were defined as: very low birth weight (VLBW ≤ 2000g); moderate low birth weight (MLBW >2000 g and ≤ 2500 g); and normal birth weight (NBW > 2500 g). Infants were tested for HIV-1 infection at birth, at weeks 1, 2, 4, 10 and 14, and at months 6, 9 and 12. Superior efficacy of SWEN relative to SD is indicated exclusively in VLBW and MLBW with greatest relative SWEN efficacy in VLBW.