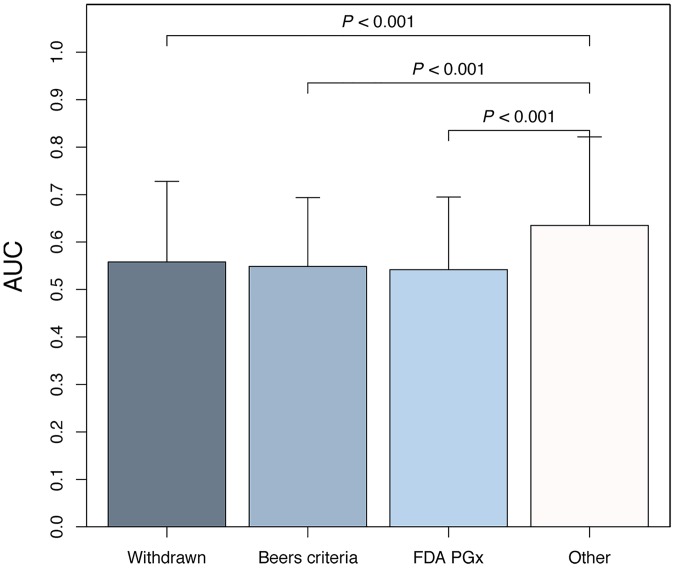
Fig 3. Comparison of population deleteriousness scores between withdrawn, precautionary, and other drugs.
Drugs withdrawn from the market (AUC = 0.558 ±0.170), precautionary drugs according to the Beers criteria (AUC = 0.549 ±0.153), and drugs labeled by the US FDA with phamacogenomic information (AUC = 0.542 ±0.145) exhibited significantly lower AUC values than other drugs (AUC = 0.635 ±0.187; P < 0.001, one-way ANOVA followed by post-hoc Tukey-tests). In contrast, the difference between the three withdrawn/precautionary drug groups did not reach statistical significance (P > 0.05). AUC, area under the drug deleteriousness score curve; FDA PGx, FDA-approved drugs with pharmacogenomic information on drug labels.