Abstract
Background
Ultrasound-assisted examination of the cardiovascular system with focused cardiac ultrasound by the treating physician is non-invasive and changes diagnosis and management of patient’s with suspected cardiac disease. This has not been reported in a general practice setting.
Aim
To determine whether focused cardiac ultrasound performed on patients aged over 50 years changes the diagnosis and management of cardiac disease by a general practitioner.
Design and setting
A prospective observational study of 80 patients aged over 50years and who had not received echocardiography or chest CT within 12months presenting to a general practice.
Method
Clinical assessment and management of significant cardiac disorders in patients presenting to general practitioners were recorded before and after focused cardiac ultrasound. Echocardiography was performed by a medical student with sufficient training, which was verified by an expert. Differences in diagnosis and management between conventional and ultrasound-assisted assessment were recorded.
Results and conclusion
Echocardiography and interpretation were acceptable in all patients. Significant cardiac disease was detected in 16 (20%) patients, including aortic stenosis in 9 (11%) and cardiac failure in 7 (9%), which were missed by clinical examination in 10 (62.5%) of these patients. Changes in management occurred in 12 patients (15% overall and 75% of those found to have significant cardiac disease) including referral for diagnostic echocardiography in 8 (10%), commencement of heart failure treatment in 3 (4%) and referral to a cardiologist in 1 patient (1%).
Routine focused cardiac ultrasound is feasible and frequently alters the diagnosis and management of cardiac disease in patients aged over 50years presenting to a general practice.
Keywords: echocardiography, diagnosis, echocardiography and management, feasibility, hand-carried ultrasound
Introduction
Patients frequently present to their general practitioner (GP) with signs and symptoms consistent with heart failure or valvular disease. Significant heart failure and aortic stenosis may become severe without symptoms (1, 2) and their incidence is increasing (3). Physical examination forms an integral part of clinical assessment of cardiac disease and helps direct appropriate tests for definitive diagnosis, but has poor diagnostic accuracy, missing or over-calling clinically important cardiac pathology approximately 50% of the time when compared with transthoracic echocardiography (TTE) in both the acute (4, 5) and outpatient (6, 7) settings. TTE is a useful and frequently performed test for diagnosis or exclusion of structural heart disease, but is a limited resource. Strategies to improve the availability of TTE to GPs are not able to solve this problem (8). Physicians with brief training are now performing their own TTE at the bedside to improve their initial clinical assessment, which improves the initial diagnostic accuracy and influences clinical management (9). This type of TTE has been recognised and defined by the American Society of Echocardiography as focused cardiac ultrasound (FCU) – “a focused examination of the cardiovascular system performed by a physician by using ultrasound as an adjunct to the physical examination to recognise specific ultrasonic signs that represent a narrow list of potential diagnoses in specific clinical settings” (10). The rapid emergence of this practice has been driven in part by the reduction in size and cost of ultrasound equipment and with the realisation that the skills required to perform FCU can be taught to non-cardiologists (6), including medical students. It is possible that general practitioners could perform their own FCU to facilitate earlier and more accurate detection of cardiac disease, which has increasing prevalence. However, there are few data on its use in the general practice setting (11).
The aim of this study was to identify the influence of FCU on the diagnosis and management of patients aged more than 50years presenting to general practice, regardless of whether they were symptomatic or asymptomatic of heart disease. Secondary aims included the feasibility of FCU and frequency of cardiac disease detected in this setting.
Methods
Study design
This prospective observational study was conducted with approval from the University of Melbourne Human Research Ethics Committee and conducted at the Nillumbik Medical Centre and the Research Medical Centre.
Selection of subjects
The medical student researcher screened patients who were waiting in the general practice waiting room for eligibility for enrolment in the study (convenience sampling) between March and June 2014 (Fig. 1). After obtaining written consent from eligible patients, the researcher performed FCU according to the described protocol. Exclusion criteria included TTE or chest CT in the preceding 12months.
Figure 1.
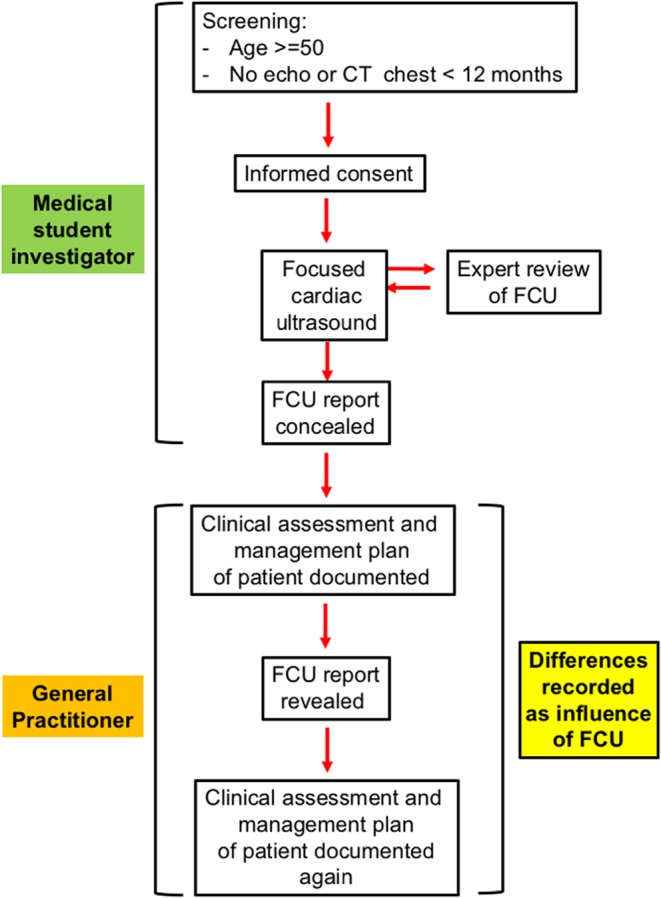
Study conduct. TTE, transthoracic echocardiography.
The image quality and findings were recorded on a standardised report form (Supplementary Fig. 1, see section on supplementary data given at the end of this article), which was initially concealed from the GP. After their conventional clinical assessment (patient history, clinical examination and review of medical history and investigations), the GP recorded their diagnosis and management plans in a standardised form before and after being shown the FCU report. Any change in diagnosis or management plan was deemed to be due to the FCU.
Measurements
The medical student investigator received training in FCU before the study consisting of 20h of interactive e-Learning (tutorials and case studies), and two days of supervised practice on human models without cardiac pathology ((iHeartScan course (6)). The medical student then practiced FCU on patients presenting to general practice. An expert critiqued the quality and interpretation of images produced by the medical student until the student was deemed competent to perform and interpret FCU independently. This was achieved after 40 FCU studies.
FCU was performed using an M-turbo (SonoSite, Bothwell, Andover, MA, USA) echocardiography machine and a 5-1MHz TTE probe, and was conducted according to the iHeartScan protocol (Haemodynamic Echocardiography Assessment in Real Time) (6, 12). This is designed to take less than 10min to perform using pattern recognition of two-dimensional and colour flow Doppler images, enabling convenient point-of-care use. This protocol has been demonstrated to be effective in screening for clinically significant cardiac pathology by anaesthetists (4, 6) in a time-efficient manner, without causing a delay in patient management (13). Clinically important cardiac pathology was defined as either left ventricular systolic or diastolic dysfunction, right ventricular systolic dysfunction, moderate or severe valve stenosis or regurgitation (14, 15), or pericardial effusion of greater than 0.5cm as defined previously (16). Left ventricular systolic dysfunction is defined as systolic fractional reduction in LV internal dimension less than 24% or LV end diastolic area less than 50%. Left ventricular diastolic dysfunction is defined as normal LV systolic function with raised left atrial pressure, which correlates with fixed curvature of the interatrial septum towards the right atrium, as demonstrated by Haji and coworkers (17). Right ventricular dysfunction is defined with FCU as dilation of the RV end diastolic area to greater than two-thirds of the LV end diastolic area and reduced RV free-wall motion with or without flattening of the interventricular septum. As sub-types of ventricular failure classified with echocardiography may present with similar clinical symptoms and signs, they were grouped together as ‘cardiac failure’ to enable a comparison of FCU with clinical diagnosis, as reported previously (4). The use of haemodynamic assessment is more relevant to anaesthesia and critical care settings as patients presenting to general practice are not usually in shock. However, the haemodynamic state was assessed with FCU by categorisation into normal, empty, vasodilated, LV systolic and/or diastolic failure, or RV failure, as described previously (16) using the assessment of LV and RV volume and function. Clinically insignificant findings included mild valvular stenosis or regurgitation or mild reduction in systolic ventricular function. The quality of imaging of the FCU was rated as interpretable or non-interpretable based on the ability to perform the complete protocol (all fields completed in the report form) from any of the three acoustic windows. Digital images were reviewed off-line for accuracy by an expert in echocardiography (CR) and the report was adjusted if required before the GP acted on the findings.
Outcomes
The primary endpoint of the study was the incidence of clinically important changes in cardiac diagnosis and management. FCU was considered the gold standard in diagnosis. Secondary endpoints included the incidence of clinically significant cardiac pathology identified by FCU, changes in management, and the image quality and accuracy of interpretation of FCU by the researcher.
Statistical analysis
The sample size was estimated using the binomial proportion confidence interval of a change of 30% of change in management (P 0.3) from before to after ultrasound examination. For a 95% confidence interval, width interval of 5%, P of 0.3 and significance set at P = 0.05, the sample size calculated was 80. Endpoints are described as frequency data without statistical comparisons. Data were coded and stored in Microsoft Excel 2010 and analysed using SPSS V21 (SPSS).
Results
Of 90 patients who were screened, seven were not included: three patients refused, three patients had insufficient time for FCU and one patient was incapable of providing consent. Of the 83 patients who were included, three were excluded: one patient was found to have received a formal TTE within 12months, one patient received a CT chest within 12months, and the GP failed to comply with the protocol in one patient. There was full data for analysis of the remaining 80 patients.
The mean age was 66 (s.d. 13.1) years and 59% were female. Patient co-morbid diseases included cigarette smoking (42.5%), hypertension (47.5%), ischaemic heart disease (12.5%), diabetes mellitus (8.8%), chronic obstructive pulmonary disease (7.5%), interstitial lung disease (7.5%) and cardiac failure (7.5%). One patient had aortic stenosis.
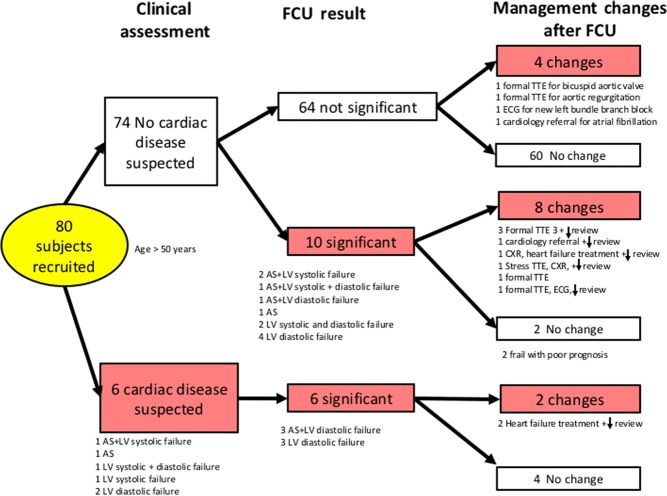
FCU was performed and interpretable in at least one acoustic window in all 80 patients. Cardiac pathology identified with FCU and the influence on diagnosis and management are summarised in Fig. 2. Significant cardiac pathology (defined in the methods) was detected with FCU in 20%, which were missed by clinical examination in 12.5% and included aortic stenosis 11% and cardiac failure in 9% of patients. FCU also confirmed aortic stenosis in one patient with a history of aortic stenosis.
Figure 2.
Diagnosis and management changes. AS, aortic valve stenosis; CXR, chest X-ray; ECG, electrocardiogram; LV, left ventricle; TTE, transthoracic echocardiogram, review ↓: decreased interval for next review by the GP.
FCU resulted in changes in patient management by the GP in 15% of patients. Of these patients, FCU revealed cardiac pathology in 62.5%. Changes included referral for diagnostic TTE (10%), commencement of heart failure treatment (4%) and referral to a cardiologist (1%). There were two patients where FCU resulted in a new cardiac diagnosis (combined aortic stenosis and cardiac failure) that had no change in management due to the decision from the GP that medical intervention was not appropriate due to poor patient prognosis.
Agreement between the investigator and the expert in the presence or absence of clinically important cardiac pathology (as defined above) occurred in 89%. In the 9 (11%) patients in whom there was disagreement in FCU interpretation between the investigator and expert, the investigator reported presence of significant pathology that was not reported as present by the expert, including right ventricular dysfunction (6%), aortic stenosis with raised left atrial pressure (2.5%), and left ventricular systolic dysfunction (1%). However, the expert reviewer deemed the differences clinically unimportant and no significant cardiac pathology was missed by the medical student. The medical management was also unaffected as the treating GP received the revised report.
Discussion
Screening of patients aged over 50years attending a GP with FCU by a novice was feasible, identified significant cardiac pathology in 20%, which was frequently missed clinically, resulting in frequent changes in patient management such as earlier referral for confirmatory echocardiography and treatment of heart failure. The most common pathologies missed by clinical examination were aortic stenosis and cardiac failure; They have a prevalence of 2–3% in the general population, which rises to 10–20% in the population aged 75years or more (1, 2). The prevalence is likely to worsen as the mean age increases. Under-detection leads to preventable morbidity and mortality (1, 2), and require echocardiography for diagnosis, as clinical assessment is unreliable (4). Widespread use of FCU in general practice may lead to earlier detection and treatment of these common but serious conditions. Additionally, echocardiography is able to differentiate systolic from diastolic heart failure (heart failure with normal ejection fraction), which accounts for more than 50% of presentations, contributes to poor prognosis, and has a different treatment to systolic heart failure (18, 19).
This study confirms other reports that a novice can rapidly acquire the skills required to perform and interpret FCU (4, 6, 20, 21, 22), with clinically acceptable accuracy. Although learning FCU requires a significant and sustained effort, it is likely that the average general practitioner will be able to achieve this level of competency.
There are an increasing number of medical and surgical specialties that are adopting this skill, such as anaesthesia, critical care, emergency medicine, respiratory medicine, internal medicine and paramedics (ambulance). Trainees in these specialties are able to learn FCU during their training despite a full curriculum and busy clinical workload. Training courses in FCU are increasingly available (9), and high fidelity FCU simulators with an increasing volume of on-line resources enable learning FCU when convenient and not encroaching on clinical practice (23). In a recent survey of medical schools in USA, 62% reported that ultrasound is taught in the curriculum (24). It is likely that new generations of general practitioners will already have learned the skill before entering general practice.
Attempts to improve GP’s access to existing TTE services have also found a high rate of important cardiac pathology. The open-access echocardiography scheme allowed GP’s access to TTE laboratories at 133 hospitals in the UK (25, 26, 27) and generated an extra 2343 TTE studies over 2years, which identified significant cardiac pathology in 29% of patients (8). Gillespie and Pringle reported significant cardiac pathology in 29% of patients from a qualified TTE technician located in the general practice (11). The authors of both studies concluded that expansion of TTE in general practice increases the detection of clinically important pathology. However, it is unlikely that either of these models of service is scalable as they rely on existing cardiology services that are already overstretched. This is in contrast to the results of our study, which may be scalable if GPs are able to perform their own TTE. Cost of the equipment is now not prohibitive, with a hand-held unit currently costing as little as GBP 9000.
Limitations of this study include the observational nature of the design, which was aimed at establishing proof of concept rather than the effect on patient outcome. Although a control group (no TTE) was not used, the patients represented their own controls, with diagnosis and management plans recorded before and after receiving echocardiography information. Changes in management due to the FCU mostly involved referral for a comprehensive TTE to confirm the findings on FCU, which may seem to be simply doubling up of services. However, without FCU it was demonstrated that clinical suspicion was absent, and therefore the effect of the FCU was to potentially detect the condition earlier, potentially prompting earlier treatment. Another limitation in this study is that although the researcher performing the FCU was deemed to be proficient in this technique, to ensure the quality of interpretation, an expert reviewed the images, which may not be easy to achieve in all GP practices.
Although routine screening is likely to be difficult to be implemented widely in general practice as many GPs are under pressure with workload, it is more likely to be successful in targeted screening of patients at increased risk of cardiac disease. This was demonstrated in the audit of 2343 TTEs performed in the GP setting by the open access scheme reported by Chambers and coworkers (8). The most common indications for TTE in the GP setting in this study were murmur, suspected heart failure (only with elevated B-type natriuretic peptide), abnormal ECG, hypertension and cardiomegaly. Patient throughput is important in general practice and the benefits of early detection of cardiac disease with FCU, such as early treatment, need to be justified by the additional time to implement FCU in clinical practice.
Conclusion
Routine FCU changes clinical diagnosis and management in a substantial proportion of patients aged over 50years presenting to general practice.
Supplementary data
This is linked to the online version of the paper at http://dx.doi.org/10.1530/ERP-16-0026.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector. Sonosite (Fujifilm) provided a loan of echocardiography equipment. There were no external sources of funding and the authors had full access to all of the data (including statistical reports and tables) in the study.
Statement of consent
Consent has been obtained from each patient or subject after full explanation of the purpose and nature of all procedures used.
Acknowledgements
The authors acknowledge the contributions from the GPs, staff and patients at the Nillumbik and Research Medical Centres.
References
- 1.Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. 2006. Burden of valvular heart diseases: a population-based study. Lancet 368 1005–1011. ( 10.1016/S0140-6736(06)69208-8) [DOI] [PubMed] [Google Scholar]
- 2.Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole-Wilson PA, A StrÖmberg, van Veldhuisen DJ, D Atar, AW Hoes, et al. 2008. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). European Heart Journal 29 2388–2442. ( 10.1093/eurheartj/ehn309) [DOI] [PubMed] [Google Scholar]
- 3.Lauer MS. 2012. Advancing cardiovascular research. Chest 141 500–505. ( 10.1378/chest.11-2521) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canty DJ, Royse CF, Kilpatrick D, Bowman L, Royse AG. 2012. The impact of focused transthoracic echocardiography in the pre-operative clinic. Anaesthesia 67 618–625. ( 10.1111/j.1365-2044.2012.07074.x) [DOI] [PubMed] [Google Scholar]
- 5.Vignon P, Mentec H, Terre S, Gastinne H, Gueret P, Lemaire F. 1994. Diagnostic accuracy and therapeutic impact of transthoracic and transesophageal echocardiography in mechanically ventilated patients in the ICU. Chest 106 1829–1834. ( 10.1378/chest.106.6.1829) [DOI] [PubMed] [Google Scholar]
- 6.Canty DJ, Royse CF, Kilpatrick D, Bowyer A, Royse AG. 2012. The impact on cardiac diagnosis and mortality of focused transthoracic echocardiography in hip fracture surgery patients with increased risk of cardiac disease: a retrospective cohort study. Anaesthesia 67 1202–1209. ( 10.1111/j.1365-2044.2012.07300.x) [DOI] [PubMed] [Google Scholar]
- 7.Kimura BJ, Shaw DJ, Agan DL, Amundson SA, Ping AC, DeMaria AN. 2007. Value of a cardiovascular limited ultrasound examination using a hand-carried ultrasound device on clinical management in an outpatient medical clinic. American Journal of Cardiology 100 321–325. ( 10.1016/j.amjcard.2007.02.104) [DOI] [PubMed] [Google Scholar]
- 8.Chambers J, Kabir S, Cajeat E. 2014. Detection of heart disease by open access echocardiography: a retrospective analysis of general practice referrals. British Journal of General Practice 64 e105–e111. ( 10.3399/bjgp14x677167) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Royse CF, Canty DJ, Faris J, Haji DL, Veltman M, Royse A. 2012. Core review: physician-performed ultrasound: the time has come for routine use in acute care medicine. Anesthesia & Analgesia 115 1007–1028. ( 10.1213/ANE.0b013e31826a79c1) [DOI] [PubMed] [Google Scholar]
- 10.Spencer KT, Kimura BJ, Korcarz CE, Pellikka PA, Rahko PS, Siegel RJ. 2013. Focused cardiac ultrasound: recommendations from the American Society of Echocardiography. Journal of the American Society of Echocardiography 26 567–581. ( 10.1016/j.echo.2013.04.001) [DOI] [PubMed] [Google Scholar]
- 11.Gillespie ND, Pringle S. 1998. A pilot study of the role of echocardiography in primary care. British Journal of General Practice 48 1182. [PMC free article] [PubMed] [Google Scholar]
- 12.Faris JG, Veltman MG, Royse CF. 2009. Limited transthoracic echocardiography assessment in anaesthesia and critical care. Best Practice & Research Clinical Anaesthesiology 23 285–298. ( 10.1016/j.bpa.2009.02.008) [DOI] [PubMed] [Google Scholar]
- 13.Canty DJ, Royse CF, Kilpatrick D, Williams DL, Royse AG. 2012. The impact of pre-operative focused transthoracic echocardiography in emergency non-cardiac surgery patients with known or risk of cardiac disease. Anaesthesia 67 714–720. ( 10.1111/j.1365-2044.2012.07118.x) [DOI] [PubMed] [Google Scholar]
- 14.Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, Iung B, Otto CM, Pellikka PA, Quinñones M, et al. 2009. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. Journal of the American Society of Echocardiography 22 1–23. ( 10.1016/j.echo.2008.11.029) [DOI] [PubMed] [Google Scholar]
- 15.Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, Nihoyannopoulos P, Otto CM, Quinones MA, Rakowski H, et al. 2003. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. Journal of the American Society of Echocardiography 16 777–802. ( 10.1016/S0894-7317(03)00335-3) [DOI] [PubMed] [Google Scholar]
- 16.Royse CF. 2009. Ultrasound-guided haemodynamic state assessment. Best Practice & Research Clinical Anaesthesiology 23 273–283. ( 10.1016/j.bpa.2009.02.009) [DOI] [PubMed] [Google Scholar]
- 17.Haji DL, Ali MM, Royse A, Canty DJ, Clarke S, Royse CF. 2014. Interatrial septum motion but not Doppler assessment predicts elevated pulmonary capillary wedge pressure in patients undergoing cardiac surgery. Anesthesiology 121 719–729. ( 10.1097/ALN.0000000000000392) [DOI] [PubMed] [Google Scholar]
- 18.Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite-Moreira AF, et al. 2007. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. European Heart Journal 28 2539–2550. ( 10.1093/eurheartj/ehm037) [DOI] [PubMed] [Google Scholar]
- 19.Vasan RS, Levy D. 2000. Defining diastolic heart failure: a call for standardized diagnostic criteria. Circulation 101 2118–2121. ( 10.1161/01.CIR.101.17.2118) [DOI] [PubMed] [Google Scholar]
- 20.Frederiksen CA, Juhl-Olsen P, Nielsen DG, Eika B, Sloth E. 2012. Limited intervention improves technical skill in focus assessed transthoracic echocardiography among novice examiners. BMC Medical Education 12 65 ( 10.1186/1472-6920-12-65) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liebo MJ, Israel RL, Lillie EO, Smith MR, Rubenson DS, Topol EJ. 2011. Is pocket mobile echocardiography the next-generation stethoscope? A cross-sectional comparison of rapidly acquired images with standard transthoracic echocardiography. Annals of Internal Medicine 155 33–38. ( 10.7326/0003-4819-155-1-201107050-00005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Royse CF, Seah JL, Donelan L, Royse AG. 2006. Point of care ultrasound for basic haemodynamic assessment: novice compared with an expert operator. Anaesthesia 61 849–855. ( 10.1111/j.1365-2044.2006.04746.x) [DOI] [PubMed] [Google Scholar]
- 23.Canty DJ, Royse AG, Royse CF. 2015. Self-directed simulator echocardiography training: a scalable solution. Anaesthesia and Intensive Care 43 425–427. [PubMed] [Google Scholar]
- 24.Bahner DP, Goldman E, Way D, Royall NA, Liu YT. 2014. The state of ultrasound education in U.S. medical schools: results of a national survey. Academic Medicine 89 1681–1686. ( 10.1097/ACM.0000000000000414) [DOI] [PubMed] [Google Scholar]
- 25.Fuat A, Hungin AP, Murphy JJ. 2003. Barriers to accurate diagnosis and effective management of heart failure in primary care: qualitative study. BMJ 326 196 ( 10.1136/bmj.326.7382.196) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arden C, Chambers JB, Sandoe J, Ray S, Prendergast B, Taggart D, Westaby S, Grothier L, Wilson J, Campbell B, et al. 2014. Can we improve the detection of heart valve disease? Heart 100 271–273. ( 10.1136/heartjnl-2013-304223) [DOI] [PubMed] [Google Scholar]
- 27.Colquhoun MC, Waine C, Monaghan MJ, Struthers AD, Mills PG. 1995. Investigation in general practice of patients with suspected heart failure: how should the essential echocardiographic service be delivered? British Journal of General Practice 45 517–519. [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a