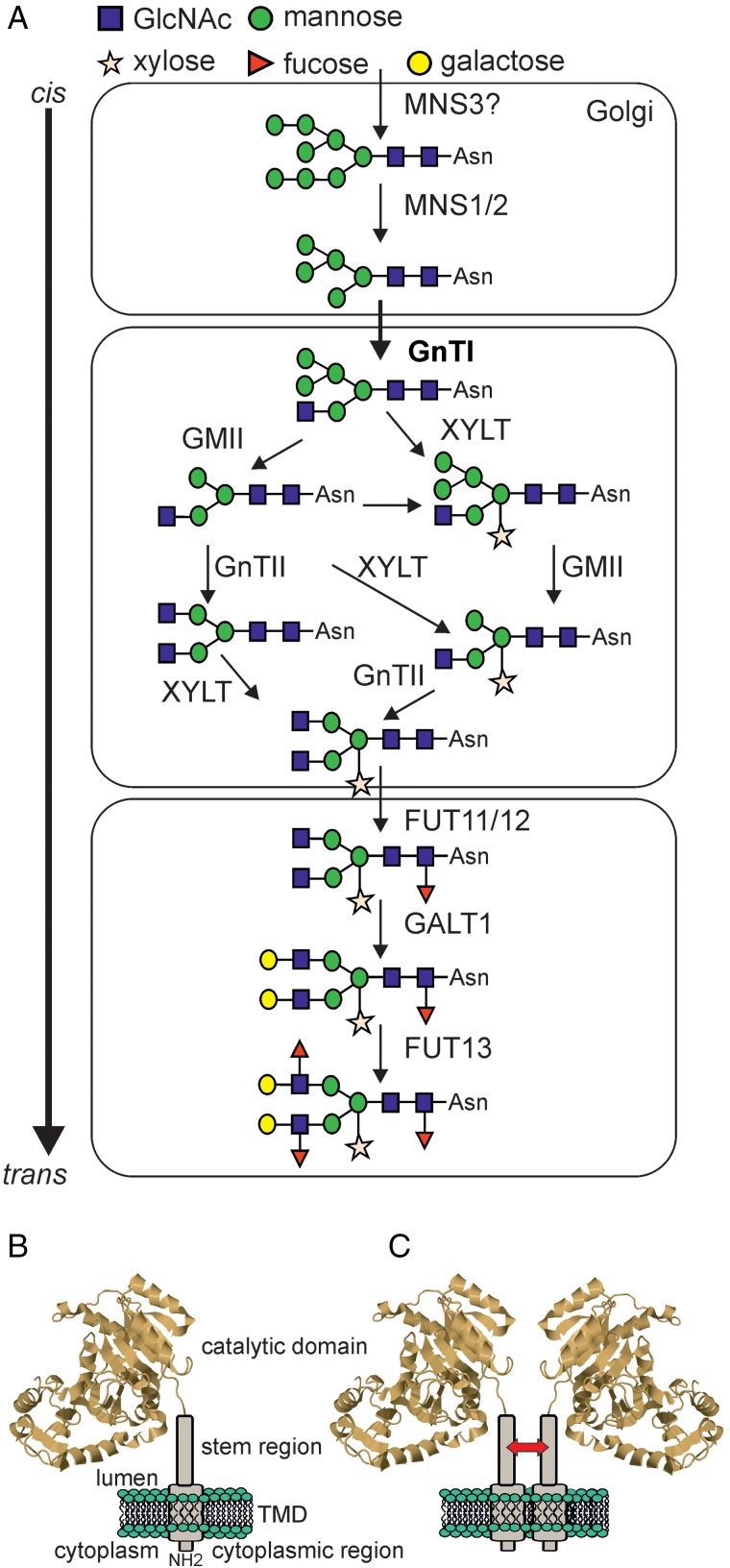
Fig. 4.
(A) Complex N-glycan formation in the Golgi apparatus. Terminal Man residues are removed by class I α-mannosidases (MNS1-3). Man5GlcNAc2 is used by β1,2-N-acetylglucosaminyltransferase I (GnTI, highlighted in bold) to initiate complex N-glycan formation. The different N-glycan processing enzymes required for the maturation of complex N-glycans in the Golgi are shown. Note, possible cargo transport processes mediated by cisternal maturation, vesicular transport or tubular connections are not indicated. Dependent on the mode of cargo transport there are differences in localization and retention of glycosylation enzymes. (B) Schematic illustration of a Golgi-resident N-glycan processing enzyme. The structure of the catalytic domain from rabbit GnTI (Unligil et al. 2000) is illustrated with N-terminal regions representing the short cytoplasmic tail, the single transmembrane domain and the stem region. (C) Golgi-resident glycosyltransferases may form homomeric or heteromeric complexes that could be important for concentration of the enzymes in different Golgi cisternae and/or for the modulation of their enzymatic activities. The arrow indicates that tobacco GnTI interacts through its stem region (Schoberer et al. 2014). This figure is available in black and white in print and in color at Glycobiology online.