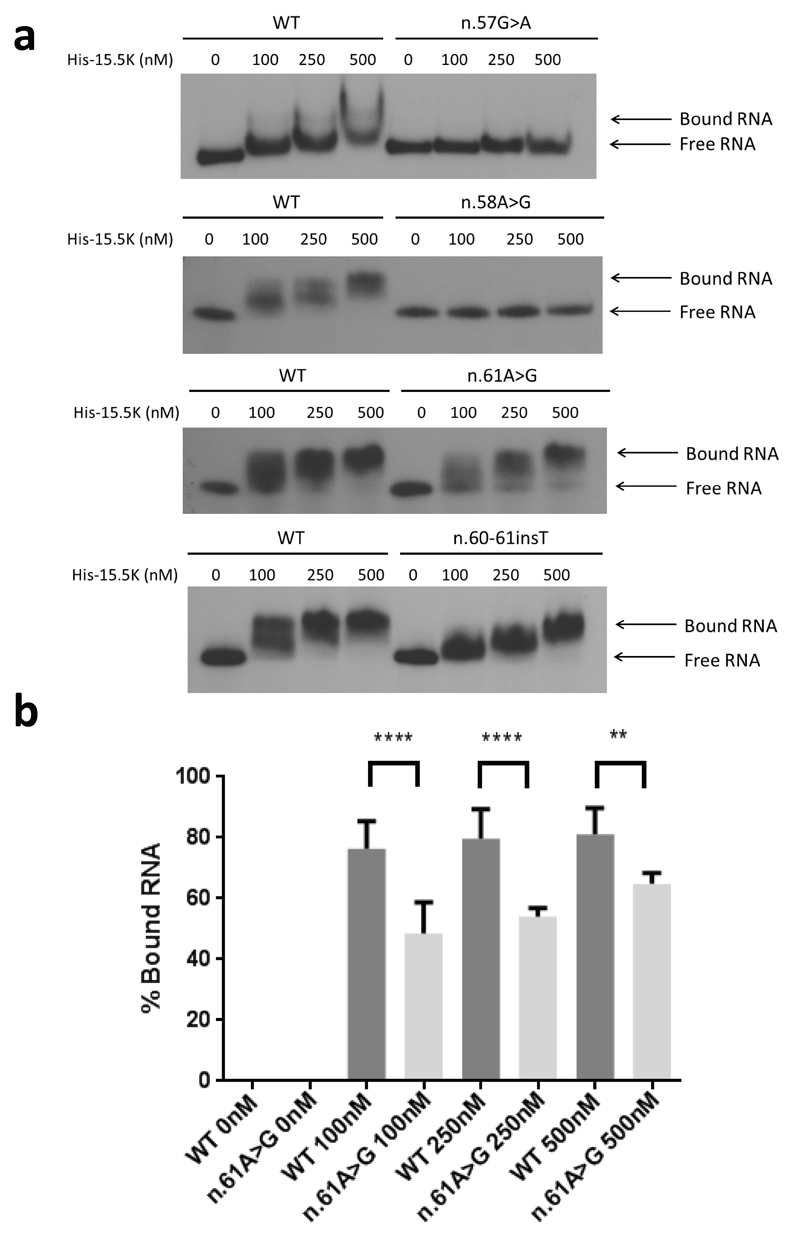
Fig. 4. Protein binding of U8 variants.
(a). Electrophoretic mobility shift assay (EMSA) using wild type (WT) and mutant 5’ end-labeled in vitro transcribed U8 snoRNA with increasing concentrations of recombinant 6His-tagged 15.5K protein (His-15.5K). The concentration of the recombinant protein is given in nM above the panels. Binding of WT RNA resulted in a shift in mobility at 100 nM. Binding of His-15.5K with n.57G>A, and n.58A>G was severely impaired. A shift in mobility could not be observed for n.57G>A or n.58A>G at protein concentrations up to 500 nM. Binding between His-15.5K and n.61A>G demonstrated a shift in mobility at 100 nM; however, this shift was less than observed in WT RNA at the same concentration, and excess free RNA can be seen at all concentrations up to 500 nM indicating that binding is impaired. Similarly binding between His-15.5K and n.60_61insT demonstrated a shift in mobility at 100 nM. This shift was less than observed in WT RNA and equivalent shift was only achieved at the highest concentration tested 500nM, indicating that binding is impaired. (b). Quantification of binding between His 15.5K and n.61A>G compared to WT RNA. The percentage of protein bound RNA is significantly decreased at all concentrations. Data are given as the mean +/- SD; n= 4 independent experiments. Data were analyzed using a one way Anova with multiple comparisons where **** = p<0.0001.