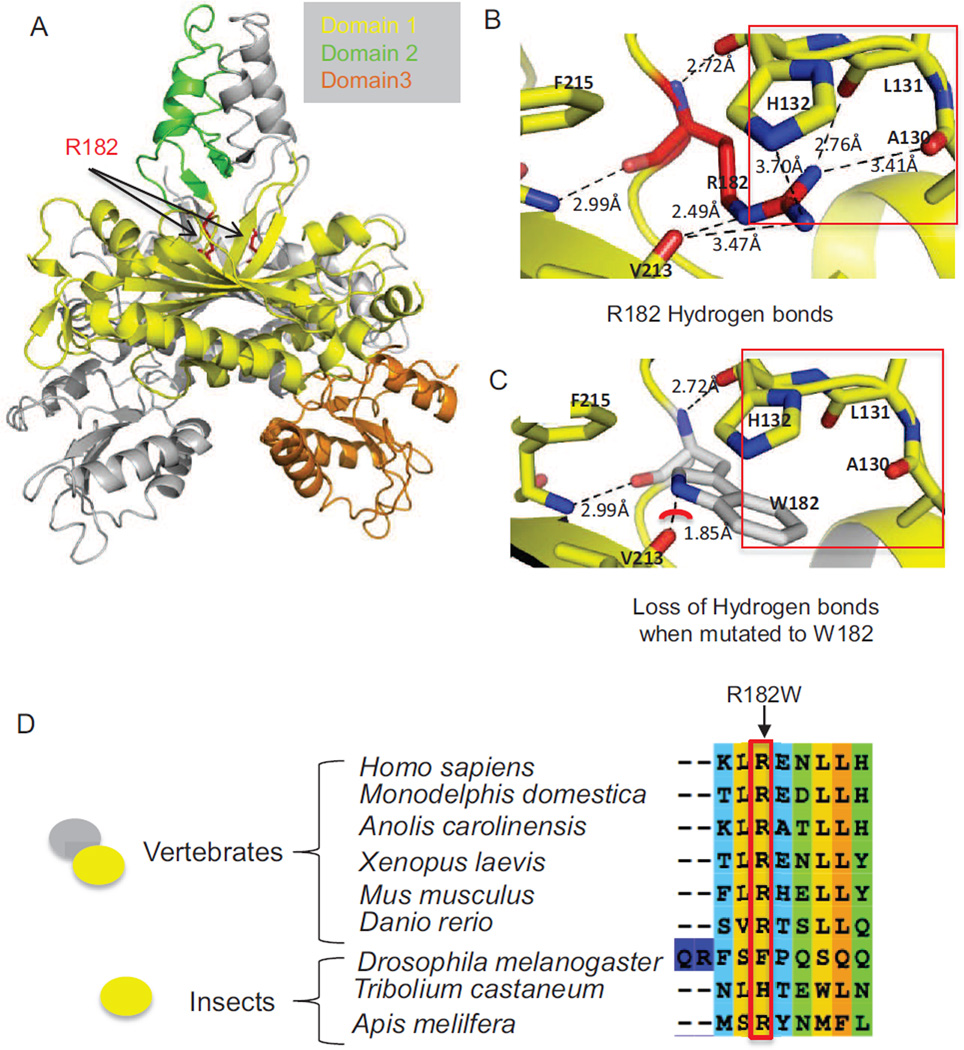
Figure 4.
Structural implications of the R182W mutation in the protein structure of human Pol γ accessory subunit, POLG2 (PDB: 2G4C) and conservation of R182 in vertebrate POLG2 sequences. (A) Overview of the POLG2 homodimer illustrating the location of R182. In gray is one monomer of POLG2 while the other monomer is broken down into domains, with domain 1 in yellow, domain 2 in green and domain 3 in orange. The arrows are pointing to Arg182, which is highlighted in red. (B) Hydrogen bonding interactions of the Arg182 side chain with the carboxy backbone of residues Val213, Leu131, and Ala130, as well as the side chain of His132, all except V213 are highlighted by the red box. (C) Loss of H-bonding when Arg182 is altered to Trp is highlighted by the red box. The red arc shows a potential steric clash between Arg182 and Val213. (D) The red-box and arrow both highlight Arg182. The oval shapes on the left indicate the oligomerization state of POLG2, which exists as a homodimer in vertebrates and a monomer in insects.