Abstract
In vivo screening of phage libraries in tumor-bearing mice has been used to identify peptides that direct phage homing to a tumor. The power of in vivo phage screening is illustrated by the recent discovery of peptides with unique tumor-penetrating properties. These peptides activate an endocytic transport pathway related to but distinct from macropinocytosis. They do so through a complex process that involves binding to a primary, tumor-specific receptor, followed by a proteolytic cleavage, and binding to a second receptor. The second receptor, neuropilin-1 (or neuropilin-2) activates the transport pathway. This trans-tissue pathway, dubbed the C-end Rule (CendR) pathway, mediates the extravasation transport through extravascular tumor tissue of payloads ranging from small molecule drugs to nanoparticles. The CendR technology provides a solution to a major problem in tumor therapy, poor penetration of drugs into tumors. Targeted delivery with tumor-penetrating peptides has been shown to specifically increase the accumulation of drugs, antibodies and nanotherapeutics in experimental tumors in vivo, and in human tumors ex vivo. Remarkably the payload does not have to be coupled to the peptide; the peptide activates a bulk transport system that sweeps along a drug present in the blood. Treatment studies in mice have shown improved anti-tumor efficacy and less damage to normal tissues with drugs ranging from traditional chemotherapeutics to antibodies, and to nanoparticle drugs.
Keywords: CendR pathway, Tumor vasculature, Synaphic targeting, Endocytosis, Integrins, Neuropilins, Cell-penetrating peptides, Nanomedicine
1. Introduction
Compounds that selectively recognize target molecules in tumors are potentially valuable reagents for targeted delivery of diagnostic and therapeutic agents into tumors (synaphic, active, or ligand-directed) targeting). There are numerous targets in tumors, both in tumor blood vessels, and on tumor cells and stromal cells within tumors. Examples include certain integrins, fibrin deposits, and tumor antigens, such as prostate specific membrane antigen (PSMA) and carcinoembryonic antigen (CEA). The targeting ligand used can be an antibody, a peptide or a natural ligand of a receptor preferentially expressed in tumors (e.g. the folate receptor). The rationale of synaphic targeting is that a drug coupled to a targeting ligand will preferentially accumulate in the tumor, resulting in greater activity and fewer side effects elsewhere in the body [1,2,3]. Despite this simple rationale and vast amount of preclinical work, progress in bringing targeted compounds into the clinic for the treatment of solid tumors has been slow. The new tumor-penetrating peptides may overcome some of the limitations of the targeting technology; they deliver drugs deep into tumor tissue and enable enhanced drug delivery even without coupling of the drug to the peptide. This review focuses on the discovery of tumor-penetrating peptides, their mechanism of action, and their use in drug delivery.
2. Discovery of tumor-penetrating peptides
2.1. In vivo phage display screening for peptides
Phage display makes use of libraries of peptides that are expressed at the surface of a phage particle, such that each phage particle expresses one peptide, and the whole library typically contains up to 10^9 different peptide sequences. The phages carrying a peptide with the desired activity are selected from the library based on their ability to bind to the desired target (unfortunately, functional screens are not possible). Sequencing the part of the phage DNA that encodes the peptide then allows identification of the peptides. In vivo phage library screening follows the same principles, but the screening is done in live animals, selecting for phages that accumulates at the desired target tissue [4,5]. The in vivo screening has a built-in negative screen in that phages that bind indiscriminately will not significantly accumulate at the target tissue because they will also bind somewhere else. This circumstance gives an advantage to those phages that only bind at the target tissue. Because the phages are a nanoparticle (T7 phage, diameter ~ 40 nm; filamentous phage dimensions, 6 nm × 900 nm), they do not readily penetrate beyond the vascular wall, and in vivo phage screening mostly probes the vasculature. Indeed, the method has revealed so much molecular heterogeneity in the vasculature of normal and diseased tissues that we have coined the term “vascular zip codes” for it [2].
Tumor blood vessels are morphologically and molecularly quite different from normal blood vessels [1], and lymphatic vessels in tumors differ from normal lymphatic vessels [6,7]. In vivo phage screening has uncovered many of these differences, and this method has also produced the first tumor-penetrating peptides, which are the topic of this review.
Using an in vivo screening procedure designed to probe tumor lymphatic vessels, we identified a peptide that specifically accumulated in tumor lymphatics and not in normal lymphatics [6]. We now know that this peptide, LyP-1, primarily accumulates in a myeloid cell/macrophage in tumors, when intravenously injected into tumor-bearing mice. Some of these cells incorporate into tumor lymphatics, causing LyP-1 accumulation in the endothelium of these vessels [8]. Endothelial cells of tumor blood vessels and tumor cells also bind LyP-1, but much less of the peptide accumulates in these cells than in tumor macrophages. The macrophages are particularly abundant in hypoxic areas of tumors, which are low on blood vessels but contain abundant, albeit dysfunctional lymphatic vasculature [9]. Remarkably, the phage carrying the LyP-1 peptide reaches these areas within minutes of systemic injection. The ability of this peptide to reach poorly vascularized parts of tumors remained a mystery for several years, until we discovered another peptide with similar tumor-penetrating properties, and set out to uncover the underlying mechanism.
The new peptide, iRGD, was identified in a screen for peptides that home to tumor metastases [10]. It is a 9-amino acid cyclic peptide (sequence: CRGDKGPDC). iRGD has the integrin-binding RGD motif, but it was immediately obvious to us that this peptide was different from standard RGD peptides; the iRGD phage and the free iRGD peptide spread much more extensively into extravascular tumor tissue than other RGD peptides, which tend to accumulate only around tumor vessels.
2.2. Molecular basis of iRGD activity and the CendR motif
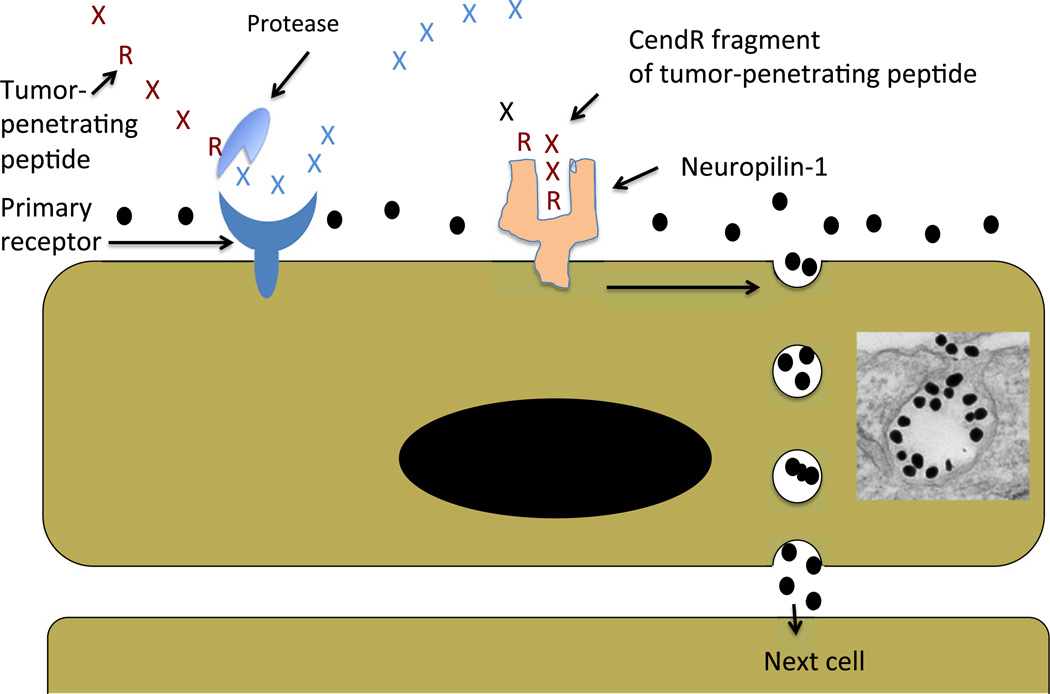
The iRGD peptide homes to tumors and accumulates in them through a 3-step process (Fig. 1): First, the integrin-binding RGD sequence motif binds to αvβ3 and αvβ5 integrins, which are specifically expressed in tumor endothelial cells. Other cells in tumors also express these integrins, which is likely to be important for the spreading of the peptide within tumor tissue, but the vascular endothelium is the gateway to the tumor for the peptide. Second, a protease cleavage event activates the CendR motif (R/KXXR/K). This protease(s) has not been identified, but is likely a furin or furin-like enzyme because the CendR motif is a preferred recognition motif for these proteases. In principle, any protease that cuts after a basic residue can activate iRGD. We have used trypsin and urokinase in vitro for this purpose [11]. The protease cleavage requires the integrin binding; a peptide that has the CendR motif but does not bind to integrins (CRGEKGPDC) is not activated. The requirement for integrin binding limits the activation of iRGD to tumors. Third, the CendR motif binds to neuropilin-1 (NRP-1) or neuropilin-2 (NRP-2), and the interaction activates an endocytotic/exocytotic transport pathway named the CendR pathway [10,11]. This pathway is responsible for the enhanced transport of drugs into tumors triggered by iRGD.
Fig. 1.
Schematic representation of the CendR trans-tissue transport pathway. A tumor-penetrating peptide with a cryptic CendR motif penetrates tumor tissue in a 3-step process: (i) The peptide binds to a primary receptor on tumor endothelium. In iRGD, the RGD motif recognizes the αvβ3/αvβ5 integrins; the primary receptor for the LyP-1 family of peptides is p32/gC1qR. (ii) The peptide is then cleaved by proteases to expose the cryptic CendR element, R/KXXR/K, at the C-terminus; and, (iii) the CendR element mediates binding to neuropilin-1 (NRP-1), inducing vascular and tissue permeability. See the text for details. Note that CendR effect enhances the tissue penetration of molecules (depicted here as a black dots) that are co-administered with the peptide [25], as well as of cargo coupled to the peptide [10]. The inset shows an electron microscopic image of a CendR endocytic vesicle that is budding from the cell surface into the cytoplasm and contains CendR peptide-coated gold nanoparticles (dark dots) (see [24]).
2.3. Family of tumor-penetrating CendR peptides
Examination of the amino acid sequence of LyP-1 (CGNKRTRGC) shows that it also contains a CendR motif, and this peptide also uses the CendR pathway [12]. The primary receptor for LyP-1 is a mitochondrial protein p32/gC1qR/HABP, which acts as a chaperone in the mitochondrial oxidative phosphorylation pathway [8,13,14]. For reasons that are not understood, p32 is expressed at the cell surface in highly activated cells, such as tumor endothelial cells, tumor macrophages, and tumor cells, whereas it remains intracellular in normal cells [8]. A truncated form of LyP-1 (CGNKRTR; tLyP-1) is also a tumor-specific CendR peptide, even though it has an active CendR motif [12]. Although RGD peptides with a basic residue following the RGD motif bind poorly to integrins [15], a peptide resembling the CendR fragment of iRGD (RGDK) has been reported to selectively home to tumors [16]. Generally, such peptides, (e.g. RPARPAR) home to all tissues, the lungs in particular, because NRP-1 is expressed in all vessels, not just tumor vessels [11]. It may be that a combination of over-expression of neuroplin-1, which is common in tumors [12,17] with even weak binding to a tumor-specific component, can render a peptide partially selective for tumor homing. tLyP-1 may be a useful peptide with characteristics complementary to those of iRGD.
Having determined the salient properties of the tumor-penetrating CendR peptides, we designed such a peptide de novo; we converted a cyclic peptide with an NGR tumor-homing motif into a cryptic CendR peptide by adding and arginine residue to create the CendR motif RNGR [18]. These examples above show that tumor-penetrating CendR peptides can bind to different primary receptors and then converge to the CendR pathway through NRP-1 binding. Two other tumor-homing peptides from our phage library screens, F3 (KDEPQRRSARLSAKPAPPKPEPKPKKAPAKK; [19] and CRGRRST [20] also contain potential CendR sequences (underlined). Whether these peptides actually act as CendR peptides has not been determined, but at least F3 shows internalization into cells [19] and has been used to target an oligonucleotide therapeutic into tumors [21].
Hajdin et al. [22] used combined in vitro/in vivo screening of 12-amino acid phage libraries to identify peptides that specifically homed to rhabdomyosarcomas. The resulting peptides contained an RXKK or RXRR motif. The authors went on to show that these peptides bound to the protease furin on the tumor cells. As discussed above, furin is the enzyme likely to process cryptic CendR peptides into active, NRP-1-binding peptides, and Hajdin et al. speculated that the furin binding may have been an intermediate step in a CendR conversion, but did not test that possibility. These results suggest that the binding of tumor-penetrating CendR peptides to furin or a furin-like protein convertase may contribute to the tumor homing, at least in cells that express high levels of such proteases.
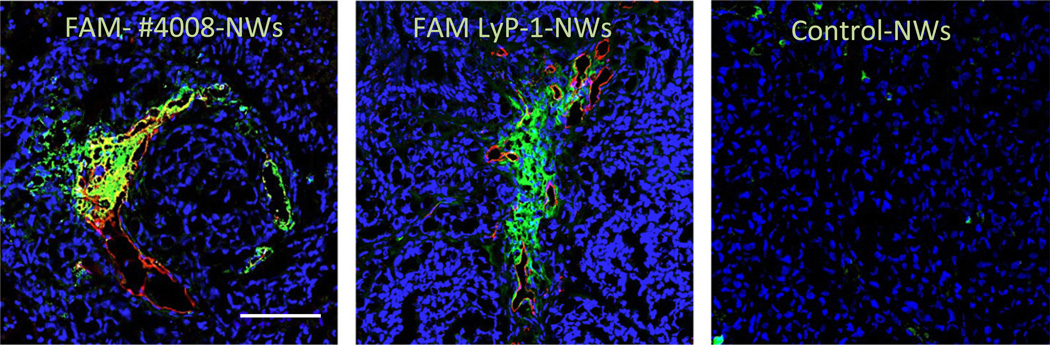
It would be potentially useful to have small molecular weight CendR compounds with drug-like chemistry. Such compounds would be more durable in vivo than peptides and could pave the way to targeted drugs that could be taken orally, rather than having to be injected. Screening of chemical libraries may be a way of identifying lead compounds toward this goal, and first efforts in this direction have been made. Our laboratory has screened for compounds that bind to the LyP-1 receptor, p32 [23]. Two compounds appeared to be specific, binding to the same site in p32 as LyP-1. One of them was studied in more detail and passed multiple validation tests, including being able to direct nanoparticle homing to tumors (Fig. 2). As expected of a compound that binds to p32, but lacks CendR properties, it did not seem to endow the nanoparticles with significant tumor-penetrating properties. It may be possible to construct compounds that fully recapitulate the tumor homing and penetrating activities of LyP-1 by combining the p32-binding compound with one that binds to NRP-1, perhaps with a self-immolating or chemically cleavable linker in between. Analogous chimeric compounds employing one of the many RGD-type compounds available with an NRP-1 binder could produce an iRGD mimic.
Fig. 2.
Nanoparticles functionalized with the p32-binding compound #4008 home to p32-positive breast tumors. OrthotopicMCF10Ca1A breast tumors from mice injected with iron oxide NPs (nanoworms; NWs) coated with FAM-#4008 [23], FAM-LyP-1, or FAM-labeled inert control peptide. The NWs were allowed to circulate for 5 h, the mice were perfused through the heart with PBS, and tumors were collected and sectioned, Confocal images of the tumors are shown (green, NWs; red, the receptor, p32; blue, nuclei). Scale bar = 100 µm.
3. The CendR pathway
3.1. The cell biology of the CendR pathway
The CendR pathway has been partially characterized. The pathway starts with an endocytosis step that is distinct from all known endocytosis pathways. The CendR endocytic vesicles most closely resemble macropinocytotic vesicles, but unlike classical macropinocytosis, the CendR pathway is receptor (neuropilin)-initiated [24]. Another significant difference to macropinocytosis is that the CendR pathway is primed for increased activity by nutrient deprivation of cells and tissues and is less responsive during abundant nutrient availability. The nutrients exert their effects on the CendR pathway through the central nutrient sensor mTOR. The mTOR regulation of the CendR pathway appears to be mostly mediated through regulation of NRP-1 expression in cells. While nutrient deficiency primes the pathway to greater activity, the actual activation of the pathway still needs to be triggered through NRP-1. These findings suggest a physiological role in supporting the survival of tissues at times of poor circulation. One corollary of the mTOR regulation of CendR is that iRGD may not be optimally active in delivering drugs to tumors with high level of mTOR activity. Combining iRGD with the mTOR inhibitor rapamycin would likely boost the efficacy of CendR-based delivery in these tumors, as rapamycin has been shown to increase the activity of the CendR pathway [24].
The endocytic vesicles that form when the CendR pathway is triggered are large, with an average diameter of about 200 nm, and can accommodate a considerable volume of extracellular fluid. If that fluid contains a drug, it will be swept into the pathway, and payloads as large as nanoparticles also readily find room in these vesicles. This circumstance explains the remarkable feature of the CendR system is that, in addition to covalently attached payloads, it will transport payloads co-administered with the peptide, but not attached to it (the “by-stander effect”). As discussed below, a number of laboratories have used iRGD is this manner to enhance drug delivery in preclinical studies [25,26,27,28].
The spreading of iRGD payloads in tumor tissue implies delivery of the payload from one cell to another. CendR is an active transport pathway; it requires energy and is much faster in tissue than what could be explained on the basis of diffusion [25]. Cell-to-cell transport of CendR cargo has been directly demonstrated in cultured tumor cell and endothelial cell spheres [24]. However, while the initial endocytic process is fairly well understood, little information is available on the route and molecular mechanisms of the cell-to-cell transport. Exosomes released from cells that have taken up CendR payload contain the payload, suggesting exosome transport as a mechanism for the cell-to-cell transport. Silver nanoparticles were transported from one cell to another even in the presence of a membrane-impermeable compound that dissolves the nanoparticles, which agrees with the notion that the CendR payload is protected by a biological membrane, such as that of exosomes. Another interesting possibility, entirely speculative at this point, is transport through micro- or nanotubes. It has been shown that tumor cells form tube-like conduits among themselves and to endothelial cells, and that that cellular macromolecules can be transported from one cell to another through these conduits [29,30,31]. The transport though these tube conduits has been found to be more effective than exosome transport, at least over short distances [30], so this mechanism could explain the effectiveness and speed of the trans-tissue transport by the CendR pathway [25].
3.2. Cell-penetrating peptides and the CendR pathway
The relationship between the tumor-penetrating peptides and the so-called cell-penetrating peptides deserves comment. Peptides that are internalized into cells are commonly referred to as cell-penetrating peptides. There are two main classes of such peptides: hydrophobic and cationic. The cationic peptides include the prototypic cell-penetrating peptides Tat and penetratin [32,33,34]. A herpes virus protein, VP22, has been shown to be capable of both entering and exiting cells [35,36] and resembles the tumor-penetrating peptides in this regard. Whether the other cell-penetrating peptides possess this tissue-penetrating function is unclear. A major limitation of these peptides as delivery vehicles is that they are not selective; they enter into all cells.
The cationic cell-penetrating peptides, including Tat, are rich in basic amino acids and typically contain one or more CendR sequence motifs. Thus, they could potentially use the CendR pathway in cell entry. The Tat peptide is commonly used in payload delivery into cells both in the natural L-configuration and as a D-amino acid peptide. Other variations include extensions at the N- and C-termini of the peptide. Tat peptide that carries a small molecular weight payload, such as fluorescein, enters cells in a manner that may involve direct penetration through the cell membrane, whereas Tat with a macromolecular or nanoparticle payload uses an endocytic pathway [24,37] The entry is generally considered to be heparan sulfate dependent; cells lacking heparan sulfate do not take up Tat, and an excess of free heparan sulfate inhibits the uptake [38,39]. Whether the CendR pathway plays a role in cellular uptake of other cell-penetrating peptides containing cryptic CendR motifs, such as penetratin, remains to be determined. A heparan sulfate-binding peptide can also be cell type and tumor specific [40].
Pang et al. [39] have compared the CendR peptide and Tat entry mechanisms for macromolecular cargo. The minimal Tat peptide, YGRKKRRQRRR-COOH, has a potential CendR motif at its C-terminus. The L-variant, when linked to a protein or nanoparticle cargo, can use either the heparan sulfate or CendR pathway, depending on which receptors are available. Blocking the C-terminus with even only an amide group abolishes Tat entry to the CendR pathway. D-Tat with the same sequence does not bind to NRP-1 and does not take nanoparticles into the CendR pathway; its entry into cells is entirely heparan sulfate dependent. Conversely, the typical CendR peptides, which do not bind to heparan sulfate, only enter the CendR pathway. The endocytic vesicles containing CendR or heparan sulfate pathway cargo are morphologically similar, but only the CendR pathway is regulated by nutrient availability to cells and tissues. Thus, these two endocytic pathways are distinct. The ability of L-Tat to use either pathway should be taken into account in interpreting result obtained with this peptide.
Heparan sulfate is a ubiquitous cell surface constituent, and consequently, Tat and other cationic cell-penetrating peptides enter most types of cells. The CendR receptor, NRP1, is also widely expressed, but is often over-expressed in tumors, and active CendR peptides have been used in tumor targeting [41,42,43]. As discussed above, greater tumor specificity is achieved by employing one of the tumor-penetrating peptides, in which theNRP-1 binding CendR motif is cryptic and activated only after a different motif directs the peptide to tumors. A similar approach has been used to direct cell-penetrating peptides to tumors. Somewhat surprisingly, a tandem peptide composed of a cell-penetrating peptide and a tumor-homing peptide has in two instances been shown to have taken on the specificity of the tumor-homing peptide [44,45]. In another strategy, the cell-penetrating activity of a Tat-like peptide, poly-arginine, was blocked by attaching to it a string of negatively charged amino acids through linker. The linker included a cleavage site for a metalloprotease highly expressed in tumors or was susceptible to chemical cleavage in the tumor environment [46,47]. Cleavage of these tandem peptides caused dissociation of the blocking sequence, activating the cell-penetrating properties of the poly-arginine part. Adding a tumor-homing sequence (RGD) to the construct further increased the tumor specificity [48]. It is not clear whether these retargeted cell-penetrating peptide constructs have any tumor-penetrating properties.
Ren et al. [49] used a tandem peptide consisting of LyP-1 (or iRGD) as a tumor-penetrating peptide and transportan to provide cell-penetrating properties. The peptide was further modified with a lipid tail to enable assembly of the peptide-lipid into micellar nanocomplexes with siRNA, and to enhance endosomal escape of the siRNA. Targeted nanocomplexes containing siRNA against Id4 protein inhibited the growth of intraperitoneal ovarian cancer tumors when injected locally, and even systemic delivery of the nanocomplexes was effective. Thus, cell-penetrating and tumor-penetrating peptides can complement one another's activities; cell-penetrating peptides are more effective in endosomal escape, whereas CendR peptides provide target specificity and tissue penetration. The target specificity can include subcellular targeting; LyP-1 and other p32-binding peptides take their payload to the mitochondria of the target cells [50], a feature that has been made use of in delivering a pro-apoptotic peptide that acts on mitochondria into tumors (discussed in the next section).
4. Tumor-penetrating CendR peptides in drug delivery: general considerations
4.1. Advantages in drug delivery
Drugs typically penetrate only few cell diameters from blood vessels into extravascular tumor tissue, and as a result, parts of the tumor receive ineffective concentrations of the drug or even no drug at all [51]. The tumor cells in these parts of the tumor stay viable, and those cells exposed to low drug concentrations are likely to development resistance to the drug. Tumor-penetrating peptides address this issue. They improve drug delivery in two interdependent ways [25]: First, they increase the drug concentration in the tumor by facilitating extravasation and tumor-penetration of a drug, while drug concentration in other tissues remains unaltered. Second, they improve the distribution of the drug within the tumor. As a result the efficacy of chemotherapy is improved.
The main reasons for the poor penetration of drugs into tumors are two-fold. First, the tumor microenvironment is characterized by a dense connective tissue stroma that creates a physical barrier to the penetration of anti-cancer drugs into tumor tissue. Pancreatic cancer is an example of a tumor that typically contains abundant stroma, which is thought to be a main factor in the resistance of these tumors to chemotherapy. Second, tumors have a high interstitial pressure, presumably because their blood vessels tend to be leaky and their lymphatic vessels are poorly functional. As a result, fluid flow is away from the tumor, and that works against penetration of drugs into the tumor [52,53]. Penetration into tumor tissue is a particularly significant issue with nanoparticle drugs (Section 5). The improved activity and penetration of drugs delivered with a tumor-penetrating peptide can be made use of to improve efficacy of the standard drug dose, or one can attenuate side effects by reducing the dose.
Drug delivery with tumor-penetrating peptides may also overcome existing resistance to the drug. Administration of nab-paclitaxel (Abraxane) nanoparticles together with iRGD or with iRGD coupled to the nanoparticle surface was in both cases effective in a breast cancer xenograft model (BT474), which is essentially resistant to paclitaxel [25]. A possible reason is that the targeted drug enters the cells by a different route (endocytosis) than the non-targeted drug, which enters directly through the cell membrane [54,55].
Another major advantage of tumor-penetrating peptides is their ability to promote tumor entry and accumulation of compounds that are not conjugated to the peptide (by-stander effect). This effect is useful in a number of ways: First, it is not necessary to create a new chemical entity to target a drug by CendR peptide co-administration, as is the case when a drug is coupled to the targeting element. This greatly simplifies the path to clinical application; when the drug to be targeted is already clinically approved; only the peptide has to be validated. Second, the by-stander effect provides a way of circumventing a major limitation of synaphic targeting, the fact that the amount of any given receptor in a target tissue is usually quite low. As a consequence, the amount of drug that can be actively targeted is also low. Measurements show that the total amount of two commonly used target receptors, αv integrins and Her2, is in the order of 200 pmol per gram of a typical tumor [56]. This number is close to the number (170 pmol) one would get by assuming that a gram of tumor tissue contains 109 cells, and that they on the average express an average of 100,000 receptors per cell. Moreover, only a fraction of the total receptor is available to the targeting ligand, between 5 and 10% in the case of the two receptors measured. Most drugs require a tissue concentration substantially higher than a few pmoles per gram of tissue, even taking into account the fact that the targeted drug conjugate is more effective than the free drug. Thus, only a limited amount of a covalently coupled drug may benefit from the targeting. Third, the by-stander effect makes it possible to use the peptide and drug at their optimal concentrations, because the targeted receptor is only used to activate the CendR transport pathway, which then transports the drug into the tumor. Because of these advantages, we are focusing our efforts on bringing iRGD into the clinic in the co-administration mode. A similar effort on LyP-1, however, uses the conjugate approach because the by-stander effect of LyP-1 seems to be weaker than that of iRGD (unpublished results). The LyP-1 project uses nanoparticles, which offer their own advantages in drug targeting as discussed in Section 5.
4.2. Pharmacokinetics considerations
The by-stander effect of intravenously injected iRGD is short-lived; it peaks at 15–30 min after intravenous injection of the peptide and is largely gone at 1 h [25]. The main reason for the short duration appears to be the short half-life of the peptide in the blood, about 8min [24]. Engineering a free sulfhydryl (–SH) group into the peptide (e.g. by adding an extra cysteine residue) can be used to prolong the half-life of iRGD [24] Once the peptide with the free sulfhydryl group is injected into the circulation, the sulfhydryl forms a disulfide bond with a sulfhydryl in albumin, nearly tripling the half-life of the peptide to 22 min. However, the bystander effect required a linker between the peptide and the albumin molecule, presumably to eliminate steric hindrance by the albumin molecule of the receptor binding. There is a parallel increase in the duration of the bystander effect and effectiveness of drug delivery. The gains in half-life and activity are not inline with the half-life of albumin, which is several days. The likely reason is that the bond to albumin is labile. It will be interesting to see if other ways of extending the iRGD half-life will provide further gains. One possibility is to couple the peptide onto the surface of long-circulating nanoparticles or incorporate it into a dendrimer. Nanoparticle-coupled iRGD is more effective in inhibiting metastasis than an equivalent amount of free iRGD peptide [57]. The half-life may be less of a problem in human patients than in mouse experiments, because the logistics of prolonged infusion or multiple injections are much easier.
The efficacy of the iRGD-enhanced co-administration effect can be high, 7–40-fold when measured within 1–3 h after intravenous single-dose administration of the drug [25]. The effect persists in mouse experiments for at least 3 weeks, but ultimately becomes about 3-fold, and the same difference is seen in the improvement of the therapeutic index. The degree of iRGD enhancement appears to be the same for a small molecular weight drug (doxorubicin) and a nanoparticle drug (Abraxane, Doxil), again indicating an active transport process. It should be noted that if the EPR effect contributes to the tumor accumulation of these nanoparticle drugs, the nanoparticle alone control includes that effect, and the inclusion of iRGD in the treatment regimen provides a further increment to the tumor accumulation and anti-tumor activity of the drug.
Tumor-penetrating peptides have also been used locally, as an intratumoral injection, to promote the spreading and cellular uptake of pro-apoptotic protein-iRGD fusion protein [58] and intraperitoneally to promote doxorubicin uptake into peritoneal carcinomatosis tumors [59]. These results show that the entry of iRGD and its payloads into tumors does not have to start with the vasculature as the entry point, as is the case when the peptide is injected into the circulation.
An important question is how to determine which tumors are likely to be responsive to therapy augmented with a tumor-penetrating peptide. One possible answer is to determine the expression levels of the relevant receptors (αv integrins and NRP1 for iRGD). The expression of αvβ3 integrin is associated with angiogenesis, which is a very early event in tumorigenesis, and primary and metastatic tumors are generally positive for αv integrins [60]. The level of NRP1 expression is clearly critical to the efficacy of the CendR transport [24], and Akashi et al. [26], found a correlation between NRP-1 expression level in pancreatic adenocarcinoma tumor cells and the responsiveness of the tumor to iRGD. Interestingly, they also found that high NRP-1 expression correlated with poor prognosis in clinical patients, suggesting that iRGD might be most useful in the worst cases. Another tumor-penetrating peptide, LyP-1, which uses cell surface-expressed p32 mitochondrial protein as its primary receptor [8], also recognizes the entire spectrum of lesions in tumorigenesis, from the premalignant lymphatic niche to metastatic tumors [6,61,62].
The use of a marker such as NRP-1 is complicated by the fact that multiple cell types, including endothelial cells, fibroblasts, macrophages and tumor cells all express the relevant receptors, including NRP-1, and it is not clear what their relative importance is in the CendR transport process and as targets of the transported drug. For example, even if the endothelium is the only sensitive cell type in a tumor, substantial augmentation of anti-tumor therapy may be obtainable through vascular disruption. Moreover, the functioning of the CendR transport system requires a complex set of proteins that may or may not be expressed in a given tumor cell. For example, cells that lack the NRP-1-interacting cytoplasmic protein, GIPC1 do not take up CendR cargo, regardless of NRP-1 expression [24]. Imaging the iRGD effect in a patient before the start of therapy may be the best way of predicting response, as was done by Schmithals et al. [28]. These authors also showed, by analyzing mouse hepatocellular carcinomas ex vivo, that iRGD increased the inhibitory effect of sorafenib on cellular phosphorylation in the tumors. As new imaging methods become available, it may be possible to image such target engagement, and the effect of iRGD on it, in vivo.
4.3. On-target side effects
Potential side effects of targeted drugs, including those targeted with tumor-penetrating peptides deserve comment. Tumor-homing probes are generally not entirely specific for tumors; they are likely to bind to a small extent to normal tissues. Thus, deleterious effects on normal tissue may be revealed in toxicology analyses. However, a greater concern is that various disease processes cause up-regulation of the very target molecules employed in tumor targeting. The laboratory animals used in experiments are young and generally healthy, whereas human cancer patients are likely to suffer from other ailments, which can also become targets for the anti-cancer treatment. For example, many tumor-targeting probes bind to molecules that are up-regulated in angiogenesis, which is shared by some non-malignant conditions, including wound healing and inflammation. The molecular changes in nonmalignant and tumor-associated angiogenesis have been reported to be different [63] but the differences are likely to be quantitative rather than absolute. Another example deals with the tumor-penetrating peptide LyP-1 and other p32-binding peptides. LyP-1 effectively and specifically homes to atherosclerotic plaques [64,65]. Thus, a drug conjugated to this peptide should first be shown not to damage atherosclerotic vessels. It may not be too difficult to select a cancer drug that is compatible with, or even beneficial in another disease of a patient. For example, paclitaxel is used in preventing restenosis after angioplasty and methotrexate in treating some inflammatory diseases.
5. Tumor-penetrating nanoparticles
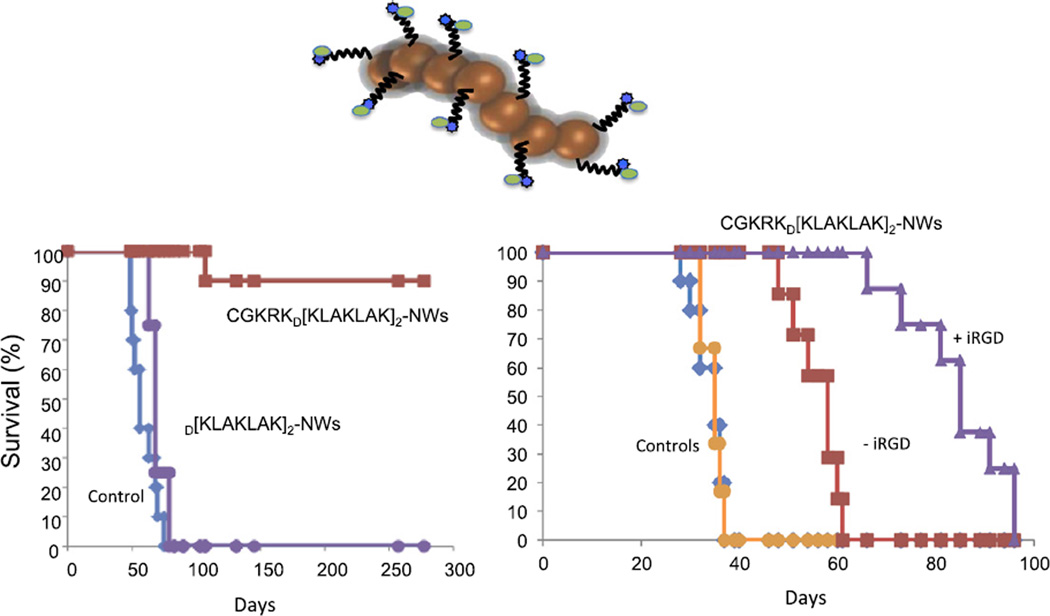
The use of nanoparticles (NPs) in medicine is receiving increasing attention. The first NP drugs are already in the clinic. Examples among cancer therapeutics include Abraxane, which is a NP composed of albumin-paclitaxel complexes, and liposomes loaded with doxorubicin (Doxil). However, these NPs are essentially passive drug delivery vehicles that do not fully exploit the potential of NPs. These clinically approved formulations primarily reach tumor tissue through nonspecific mechanisms, such as the enhanced permeability and retention (EPR) effect [66]. Nanoparticle drugs can be readily engineered to carry out additional functions that improve performance. One such function is targeted delivery. Tumor-penetrating peptides are particularly suitable for NP delivery by surface coupling. First, internalization of the peptide and its payload into cells in the tumor makes tumor homing more effective [9,67,68]. Second, these peptides can take a NP payload into the cytoplasm, which is critical, for example, in the delivery of nucleic acid-based therapeutics. Third, tumor-penetrating capabilities can enhance NP extravasation and spreading in tumor tissue. NPs, because of their size are particularly prone to be excluded from difficult-to-access parts of tumors [69] and the peptides can mitigate this problem. On the other hand, NPs are a particularly favorable cargo for homing peptides, including tumor-penetrating peptides, because multivalent presentation on the NP surface makes up for the relatively low affinity of the peptides through the avidity effect, enhancing receptor binding [2]. Moreover, drugs coupled to the surface of NPs may benefit from a similar effect. This property of NPs was made use of in designing a tumor-penetrating nanosystem that delivers as a drug a pro-apoptotic peptide, the activity of which is enhanced by a factor of 100–300 by the presentation on the surface of the NPs [50]. Other features of the nanosystem include a tumor-homing peptide that takes the proapoptotic drug peptide inside the target cells in the tumor and all the way to the mitochondria, which is where the pro-apoptotic peptide acts. Finally, iRGD given separately provides a tumor-penetrating function to the nanosystem This system has given impressive treatment results in glioblastoma [50] mouse models (Fig. 3). An inherent functional activity of a tumor-homing peptide can also be enhanced by NP presentation. The anti-metastatic activity of the iRGD peptide [70] is stronger when the peptide is on NPs than when it is used as soluble peptide [57].
Fig. 3.
Nanosystem treatment of glioblastomas in mouse models. Mice bearing lentiviral oncogene-induced (left) or transplantable (right) brain tumors were intravenously treated with a targeted nanosystem schematically depicted in the figure (see text for description of the system). Kaplan–Meier survival plots are shown. CGKRK is the homing peptide, and D(KLAKLAK)2 is the pro-apoptotic peptide. The lentiviral induced tumors were highly responsive to the nanosystem (CGKRK- D(KLAKLAK)2-NWs), with complete eradication of the tumors in almost all mice. The growth of the more aggressive transplantable tumors was delayed, and this effect was further enhanced when the iRGD peptide was co-administered with the nanosystem. Modified from [50 Agemy, et al., 2011] with permission.
6. Tumor-penetrating peptides in pre-clinical treatment studies
A number of laboratories have used iRGD, and to a lesser extent LyP-1, in tumor treatment studies using either the coupled or co-administration modes. The drugs targeted with iRGD include conventional chemotherapeutics (doxorubicin, gemcitabine), tyrosine kinase inhibitors, anti-tumor antibodies, and nanoparticle drugs, and the tumor models used range from subcutaneous and orthotopic tumors, both syngeneic transplantable and xenografts (Tables 1–3). The co-administration mode of using iRGD was initially met with skepticism, likely because the concept was novel and unprecedented. The Sugahara et al. [25] paper was selected as one of the studies to be reproduced in the Reproducibility Project [71]. Those results are not yet available. In the meantime, a number of papers have appeared that reproduce the original results (Table 2). Moreover, the mechanism of the bystander effect the co-administration is based on, a form of fluid-phase endocytosis (macropinocytosis) triggered by peptide binding to NRP-1 [24], is beginning to be understood (Section 3.1). In addition, a large number of papers have used iRGD to target coupled payloads to tumors through the same pathway (Table 3).
Table 1.
Tumor-penetrating peptides.
| Peptide | Sequence | Receptor | Target cells in tumor | Reference |
|---|---|---|---|---|
| iRGD | CRGDKGPDC | αv integrins, NRP-1/2 | Tumor fibroblasts, tumor cells | [10] |
| LyP-1 | CGNKRTRGC | p32, NRP-1/2 | Tumor endothelial cells, macrophages, tumor lymphatics, tumor cells | [8,12] |
| TT1 | CKRGARSTC | p32, NRP-1/2 | Tumor endothelial cells, macrophages, tumor lymphatics, tumor cells | [23] |
| Linear TT1 | AKRGARSTA | p32, NRP-1/2 | Tumor endothelial cells, macrophages, tumor lymphatics, tumor cells | [23]; Sharma et al., submitted |
| iNGR | CRNGRGPDC | Aminopeptidase N,NRP-1/2 | Tumor endothelial cells, other cells in tumors? | [18] |
| tLyp-1 | CGNKRTR | ?, NRP-1/2 | Tumor endothelial cells, other NRP-positive cells in tumors | [12] |
Table 3.
iRGD conjugates used in treatment studies.
| Drug-peptide conjugates | |||
|---|---|---|---|
| Reference | Peptide | Drug | Tumor model |
| [10,25] | iRGD | Nab-paclitaxel | 22Rv1 human prostate cancer and BT474 human breast cancer xenografts |
| [77] | iRGD | Paclitaxel in nanoparticles | H22 murine liver cancer |
| [78] | iRGD | Thymopentin fused to iRGD | MCF7 human breast cancer xenograft |
| [79] | iRGD | Doxorubicin in chitosan nanoparticles | H22 murine liver cancer |
| [80] | iRGD | Doxorubicin liposomes | B16 mouse melanoma |
| [81] | iRGD | Doxorubicin multilamellar liposomes | 4T1 mouse mammary cancer |
| [82] | iRGD | Paclitaxel nanoparticles | C6 rat glioma |
| [83] | iRGD | Oncolytic virus | A549 human non-small cell lung carcinoma xenograft |
| [84] | iRGD | Paclitaxel liposomes | B16 mouse melanoma |
| [85] | iRGD | Paclitaxel and surviving siRNA in nanoparticles | A549 human non-small cell lung carcinoma xenograft |
| [86] | iRGD | Doxorubicin-containing exosomes | MDA-MB-231 human breast cancer xenograft |
| [87] | iRGD | ATAP mitochondrial targeting peptide | Du145 and PC3 human prostate cancer xenografts |
| [27] | iRGD | Anti-EGFR | BGC-823 human gastric adenocarcinoma xenograft |
| [88] | iRGD | Nanomicelles containing salinomycin | HepG2 human liver cancer xenograft |
| [89] | iRGD | Microbubbles for ultrasound imaging | 4T1 mouse mammary cancer |
| [90] | iRGD | Liposomal doxorubicin | B16 mouse melanoma |
| [91] | iRGD | Paclitaxel nano-crystallite | 4T1 mouse mammary cancer |
| [92] | iRGD | Combrestatin A4 and doxorubicin in nanoparticles | HeLa human cervical cancer |
| [31] | iRGD | Sorafenib in porous silicon nanoparticles | PC3-MM2 human prostate cancer xenograft |
Table 2.
Co-administration of tumor-penetrating peptide together with drugs in treatment studies.
| Drug-peptide combinations | |||
|---|---|---|---|
| Reference | Peptide | Drug | Tumor model |
| [25] | RGD | Nab-paclitaxel, doxorubicin, doxorubicin liposomes, trastuzumab | 22Rv1 human prostate cancer and BT474 human breast cancer xenografts |
| [72] | iRGD | Cisplatin in naoparticles | A549 human non-small cell lung carcinoma xenograft |
| [26] | iRGD | Gemcitabine | Pancreatic adenocarcinoma models |
| [73] | iRGD | Gemcitabine | A549 human non-small cell lung carcinoma xenograft |
| [28] | iRGD | Doxorubicin, sorafenib | HepG2 and Hu-7 human hepatocellular carcinoma xenografts |
| [27] | iRGD | Doxorubicin | BGC-823 human gastric adenocarcinoma xenograft |
| [59] | iRGD | Intraperitoneal doxorubicin | Lovo-6-luc-1 human colon cancer and MKN45P gastric cancer xenografts |
| [74] | iRGD | Nanoparticles containing doxorubicin | 4T1 mouse mammary cancer |
| [18] | iNGR | Doxorubicin | 4T1 mouse mammary cancer |
| [75] | tLyP-1 | Paclitaxel nanoparticles | C6 rat glioma |
| [76] | tLyp-1 | Tumor targeted nanoparticles | C6 rat glioma (intracranial) |
Akashi et al. [26] studied co-administration of gemcitabine with iRGD in various pancreatic cancer mouse models. They identified transplantable, cell line-derived xenograft models that over-expressed NRP-1, and showed significantly greater tumor growth reduction with the iRGD co-administration than with gemcitabine alone. They also concluded that tumors directly transplanted into mice from patients were not significantly sensitive to the iRGD therapy. This conclusion, however, does not entirely agree with their data, as one of the three tumors tested did show a significant effect from the iRGD co-administration, and the other two were so sensitive to gemcitabine alone that there was little room for improvement. Unfortunately, the treatment was not continued to see if a statistically significant difference would have developed. The real responsiveness of human tumors to the iRGD-enhanced therapies can only be established in clinical trials, and work toward that goal is underway.
7. Conclusions and future prospects
Tumor-penetrating peptides are a class of tumor-homing peptides capable of delivering both covalently coupled and co-administered drug payloads deep into tumor tissue. As a result, the amount of drug delivered into the tumor, but not elsewhere in the body, increases, and the distribution of the drug within the tumor improves. The underlying mechanism is activation by the peptide of the CendR transport pathway, an endocytotic pathway resembling macropinocytosis that sweeps along any drug bound to the peptide or administered with it (bystander effect). Drug delivery employing tumor-penetrating peptides can solve a major problem in cancer chemotherapy, poor penetration of drugs into tumors, which limits therapeutic efficacy and creates conditions conducive to the development of drug resistance. The bystander effect also circumvents a problem that otherwise limit the efficacy of synaphic drug targeting, the low capacity of receptors in tumor tissue. Because the tumor-specific receptors are only used for CendR activation, not for capturing a drug-peptide conjugate, the capacity of the receptors is less of an issue. Further improvements will undoubtedly come from better understanding of the cell biology of the CendR system and from advancements in the design of the activating compounds Nonetheless, the first generation compounds, such as the iRGD and LyP-1 peptides are poised for studies enabling clinical trials.
Acknowledgments
I thank Dr. Eva Engvall for comments on the manuscript. Research in the author's laboratory is supported by Grants CA152327 and RO1 CA188883, and Cancer Center Support grant CA030199 from the National Cancer Institute.
Conflict of interest statement
The author is a shareholder and officer/consultant of DrugCendR LLC and EnduRx Inc., which hold certain rights to the peptides reviewed in this article.
References
- 1.Ruoslahti E, Bhatia SN, Sailor MJ. Targeting of drugs and nanoparticles to tumors. J. Cell Biol. 2010;188:759–768. doi: 10.1083/jcb.200910104. http://dx.doi.org/10.1083/jcb.200910104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruoslahti E. Peptides as targeting elements and tissue penetration devices for nanoparticles. Adv. Mater. 2012;24:3747–3756. doi: 10.1002/adma.201200454. http://dx.doi.org/10.1002/adma.201200454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yao VJ, D'Angelo S, Butler KS, Theron C, Smith TL, Marchio S, Gelovani JG, Sidman RL, Dobroff AS, Brinker CJ, Bradbury AR, Arap W, Pasqualini R. Ligand-targeted theranostic nanomedicines against cancer. J. Control. Release. 2016 doi: 10.1016/j.jconrel.2016.01.002. http://dx.doi.org/10.1016/j.jconrel.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pasqualini R, Ruoslahti E. Organ targeting in vivo using phage display peptide libraries. Nature. 1996;380:364–366. doi: 10.1038/380364a0. http://dx.doi.org/10.1038/380364a0. [DOI] [PubMed] [Google Scholar]
- 5.Teesalu T, Sugahara KN, Ruoslahti E. Mapping of vascular ZIP codes by phage display. Methods Enzymol. 2012;503:35–56. doi: 10.1016/B978-0-12-396962-0.00002-1. http://dx.doi.org/10.1016/B978-0-12-396962-0.00002-1. [DOI] [PubMed] [Google Scholar]
- 6.Laakkonen P, Porkka K, Hoffman JA, Ruoslahti E. A tumor-homing peptide with a targeting specificity related to lymphatic vessels. Nat. Med. 2002;8:751–755. doi: 10.1038/nm720. http://dx.doi.org/10.1038/nm720. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L, Giraudo E, Hoffman JA, Hanahan D, Ruoslahti E. Lymphatic zip codes in premalignant lesions and tumors. Cancer Res. 2006;66:5696–5706. doi: 10.1158/0008-5472.CAN-05-3876. http://dx.doi.org/10.1158/0008-5472.CAN-05-3876. [DOI] [PubMed] [Google Scholar]
- 8.Fogal V, Zhang L, Krajewski S, Ruoslahti E. Mitochondrial/cell-surface protein p32/gC1qR as a molecular target in tumor cells and tumor stroma. Cancer Res. 2008;68:7210–7218. doi: 10.1158/0008-5472.CAN-07-6752. http://dx.doi.org/10.1158/0008-5472.CAN-07-6752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laakkonen P, Akerman ME, Biliran H, Yang M, Ferrer F, Karpanen T, Hoffman RM, Ruoslahti E. Antitumor activity of a homing peptide that targets tumor lymphatics and tumor cells. Proc. Natl. Acad. Sci. U. S. A. 2004;101:9381–9386. doi: 10.1073/pnas.0403317101. http://dx.doi.org/10.1073/pnas.0403317101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sugahara KN, Teesalu T, Karmali PP, Kotamraju VR, Agemy L, Girard OM, Hanahan D, Mattrey RF, Ruoslahti E. Tissue-penetrating delivery of compounds and nanoparticles into tumors. Cancer Cell. 2009;16:510–520. doi: 10.1016/j.ccr.2009.10.013. http://dx.doi.org/10.1016/j.ccr.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teesalu T, Sugahara KN, Kotamraju VR, Ruoslahti E. C-end rule peptides mediate neuropilin-1-dependent cell, vascular, and tissue penetration. Proc. Natl. Acad. Sci. U. S. A. 2009;106:16157–16162. doi: 10.1073/pnas.0908201106. http://dx.doi.org/10.1073/pnas.0908201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roth L, Agemy L, Kotamraju VR, Braun G, Teesalu T, Sugahara KN, Hamzah J, Ruoslahti E. Transtumoral targeting enabled by a novel neuropilin-binding peptide. Oncogene. 2012;31:3754–3763. doi: 10.1038/onc.2011.537. http://dx.doi.org/10.1038/onc.2011.537. [DOI] [PubMed] [Google Scholar]
- 13.Fogal V, Richardson AD, Karmali PP, Scheffler IE, Smith JW, Ruoslahti E. Mitochondrial p32 protein is a critical regulator of tumor metabolism via maintenance of oxidative phosphorylation. Mol. Cell. Biol. 2010;30:1303–1318. doi: 10.1128/MCB.01101-09. http://dx.doi.org/10.1128/MCB.01101-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yagi M, Uchiumi T, Takazaki S, Okuno B, Nomura M, Yoshida S, Kanki T, Kang D. p32/gC1qR is indispensable for fetal development and mitochondrial translation: importance of its RNA-binding ability. Nucleic Acids Res. 2012;40:9717–9737. doi: 10.1093/nar/gks774. http://dx.doi.org/10.1093/nar/gks774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pierschbacher MD, Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984;309:30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- 16.Pramanik D, Majeti BK, Mondal G, Karmali PP, Sistla R, Ramprasad OG, Srinivas G, Pande G, Chaudhuri A. Lipopeptide with a RGDK tetrapeptide sequence can selectively target genes to proangiogenic alpha5beta1 integrin receptor and mouse tumor vasculature. J. Med. Chem. 2008;51:7298–7302. doi: 10.1021/jm800915y. http://dx.doi.org/10.1021/jm800915y. [DOI] [PubMed] [Google Scholar]
- 17.Bagri A, Tessier-Lavigne M, Watts RJ. Neuropilins in tumor biology. Clin. Cancer Res. 2009;15:1860–1864. doi: 10.1158/1078-0432.CCR-08-0563. http://dx.doi.org/10.1158/1078-0432.CCR-08-0563. [DOI] [PubMed] [Google Scholar]
- 18.Alberici L, Roth L, Sugahara KN, Agemy L, Kotamraju VR, Teesalu T, Bordignon C, Traversari C, Rizzardi GP, Ruoslahti E. De novo design of a tumor-penetrating peptide. Cancer Res. 2013;73:804–812. doi: 10.1158/0008-5472.CAN-12-1668. http://dx.doi.org/10.1158/0008-5472.CAN-12-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Porkka K, Laakkonen P, Hoffman JA, Bernasconi M, Ruoslahti E. A fragment of the HMGN2 protein homes to the nuclei of tumor cells and tumor endothelial cells in vivo. Proc. Natl. Acad. Sci. U. S. A. 2002;99:7444–7449. doi: 10.1073/pnas.062189599. http://dx.doi.org/10.1073/pnas.062189599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joyce JA, Laakkonen P, Bernasconi M, Bergers G, Ruoslahti E, Hanahan D. Stage-specific vascular markers revealed by phage display in a mouse model of pancreatic islet tumorigenesis. Cancer Cell. 2003;4:393–403. doi: 10.1016/s1535-6108(03)00271-x. [DOI] [PubMed] [Google Scholar]
- 21.Henke E, Perk J, Vider J, de Candia P, Chin Y, Solit DB, Ponomarev V, Cartegni L, Manova K, Rosen N, Benezra R. Peptide-conjugated antisense oligonucleotides for targeted inhibition of a transcriptional regulator in vivo. Nat. Biotechnol. 2008;26:91–100. doi: 10.1038/nbt1366. http://dx.doi.org/10.1038/nbt1366. [DOI] [PubMed] [Google Scholar]
- 22.Hajdin K, D'Alessandro V, Niggli FK, Schafer BW, Bernasconi M. Furin targeted drug delivery for treatment of rhabdomyosarcoma in a mouse model. PLoS One. 2010;5:e10445. doi: 10.1371/journal.pone.0010445. http://dx.doi.org/10.1371/journal.pone.0010445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paasonen LS, Braun SG, Kotamraju VR, Chung T, She Z-G, Sugahara KN, Yliperttula M, Wu B, Pellecchia M, Ruoslahti E, Teesalu T. New p32/gC1qR ligands for targeted tumor drug delivery. Chembiochem. 2016;17:570–575. doi: 10.1002/cbic.201500564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pang HB, Braun GB, Friman T, Aza-Blanc P, Ruidiaz ME, Sugahara KN, Teesalu T, Ruoslahti E. An endocytosis pathway initiated through neuropilin-1 and regulated by nutrient availability. Nat. Commun. 2014;5:4904. doi: 10.1038/ncomms5904. http://dx.doi.org/10.1038/ncomms5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sugahara KN, Teesalu T, Karmali PP, Kotamraju VR, Agemy L, Greenwald DR, Ruoslahti E. Coadministration of a tumor-penetrating peptide enhances the efficacy of cancer drugs. Science. 2010;328:1031–1035. doi: 10.1126/science.1183057. http://dx.doi.org/10.1126/science.1183057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akashi Y, Oda T, Ohara Y, Miyamoto R, Kurokawa T, Hashimoto S, Enomoto T, Yamada K, Satake M, Ohkohchi N. Anticancer effects of gemcitabine are enhanced by co-administered iRGD peptide in murine pancreatic cancer models that overexpressed neuropilin-1. Br. J. Cancer. 2014;110:1481–1487. doi: 10.1038/bjc.2014.49. http://dx.doi.org/10.1038/bjc.2014.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sha H, Zou Z, Xin K, Bian X, Cai X, Lu W, Chen J, Chen G, Huang L, Blair AM, Cao P, Liu B. Tumor-penetrating peptide fused EGFR single-domain antibody enhances cancer drug penetration into 3D multicellular spheroids and facilitates effective gastric cancer therapy. J. Control. Release. 2015;200:188–200. doi: 10.1016/j.jconrel.2014.12.039. http://dx.doi.org/10.1016/j.jconrel.2014.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmithals C, Koberle V, Korkusuz H, Pleli T, Kakoschky B, Augusto EA, Ibrahim AA, Arencibia JM, Vafaizadeh V, Groner B, Korf HW, Kronenberger B, Zeuzem S, Vogl TJ, Waidmann O, Piiper A. Improving drug penetrability with iRGD leverages the therapeutic response to sorafenib and doxorubicin in hepatocellular carcinoma. Cancer Res. 2015;75:3147–3154. doi: 10.1158/0008-5472.CAN-15-0395. http://dx.doi.org/10.1158/0008-5472.CAN-15-0395. [DOI] [PubMed] [Google Scholar]
- 29.Thayanithy V, Dickson EL, Steer C, Subramanian S, Lou E. Tumor-stromal cross talk: direct cell-to-cell transfer of oncogenic microRNAs via tunneling nanotubes. Transl. Res. 2014;164:359–365. doi: 10.1016/j.trsl.2014.05.011. http://dx.doi.org/10.1016/j.trsl.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Connor Y, Tekleab S, Nandakumar S, Walls C, Tekleab Y, Husain A, Gadish O, Sabbisetti V, Kaushik S, Sehrawat S, Kulkarni A, Dvorak H, Zetter B, Sengupta REES. Physical nanoscale conduit-mediated communication between tumour cells and the endothelium modulates endothelial phenotype. Nat. Commun. 2015;6:8671. doi: 10.1038/ncomms9671. http://dx.doi.org/10.1038/ncomms9671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang CF, Sarparanta MP, Makila EM, Hyvonen ML, Laakkonen PM, Salonen JJ, Hirvonen JT, Airaksinen AJ, Santos HA. Multifunctional porous silicon nanoparticles for cancer theranostics. Biomaterials. 2015;48:108–118. doi: 10.1016/j.biomaterials.2015.01.008. http://dx.doi.org/10.1016/j.biomaterials.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 32.Derossi D, Chassaing G, Prochiantz A. Trojan peptides: the penetratin system for intracellular delivery. Trends Cell Biol. 1998;8:84–87. [PubMed] [Google Scholar]
- 33.Meade BR, Dowdy SF. Exogenous siRNA delivery using peptide transduction domains/cell penetrating peptides. Adv. Drug Deliv. Rev. 2007;59:134–140. doi: 10.1016/j.addr.2007.03.004. http://dx.doi.org/10.1016/j.addr.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 34.van den Berg A, Dowdy SF. Protein transduction domain delivery of therapeutic macromolecules. Curr. Opin. Biotechnol. 2011;22:888–893. doi: 10.1016/j.copbio.2011.03.008. http://dx.doi.org/10.1016/j.copbio.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 35.Brewis ND, Phelan A, Normand N, Choolun E, O'Hare P. Particle assembly incorporating a VP22-BH3 fusion protein, facilitating intracellular delivery, regulated release, and apoptosis. Mol. Ther. 2003;7:262–270. doi: 10.1016/s1525-0016(02)00054-0. [DOI] [PubMed] [Google Scholar]
- 36.Elliott G, O'Hare P. Intercellular trafficking and protein delivery by a herpes virus structural protein. Cell. 1997;88:223–233. doi: 10.1016/s0092-8674(00)81843-7. [DOI] [PubMed] [Google Scholar]
- 37.Kaplan IM, Wadia JS, Dowdy SF. Cationic TAT peptide transduction domain enters cells by macropinocytosis. J. Control. Release. 2005;102:247–253. doi: 10.1016/j.jconrel.2004.10.018. http://dx.doi.org/10.1016/j.jconrel.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 38.Gump JM, Dowdy SF. TAT transduction: the molecular mechanism and therapeutic prospects. Trends Mol. Med. 2007;13:443–448. doi: 10.1016/j.molmed.2007.08.002. http://dx.doi.org/10.1016/j.molmed.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 39.Pang HB, Braun GB, Ruoslahti E. Neuropilin-1 and heparan sulfate proteoglycans cooperate in cellular uptake of nanoparticles functionalized by cationic cell-penetrating peptides. Sci. Adv. 2015;1:e1500821. doi: 10.1126/sciadv.1500821. http://dx.doi.org/10.1126/sciadv.1500821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Järvinen T, Ruoslahti E. Molecular changes in the vasculature of injured tissues. Am. J. Path. 2007;171:702–711. doi: 10.2353/ajpath.2007.061251. PMID: 17600129. PMCID: PMC1934529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karjalainen K, Jaalouk DE, Bueso-Ramos CE, Zurita AJ, Kuniyasu A, Eckhardt BL, Marini FC, Lichtiger B, O'Brien S, Kantarjian HM, Cortes JE, Koivunen E, Arap W, Pasqualini R. Targeting neuropilin-1 in human leukemia and lymphoma. Blood. 2011;117:920–927. doi: 10.1182/blood-2010-05-282921. http://dx.doi.org/10.1182/blood-2010-05-282921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kumar A, Huo S, Zhang X, Liu J, Tan A, Li S, Jin S, Xue X, Zhao Y, Ji T, Han L, Liu H, Zhang X, Zhang J, Zou G, Wang T, Tang S, Liang XJ. Neuropilin-1-targeted gold nanoparticles enhance therapeutic efficacy of platinum(IV) drug for prostate cancer treatment. ACS Nano. 2014;8:4205–4220. doi: 10.1021/nn500152u. http://dx.doi.org/10.1021/nn500152u. [DOI] [PubMed] [Google Scholar]
- 43.Shin TH, Sung ES, Kim YJ, Kim KS, Kim SH, Kim SK, Lee YD, Kim YS. Enhancement of the tumor penetration of monoclonal antibody by fusion of a neuropilin-targeting peptide improves the antitumor efficacy. Mol. Cancer Ther. 2014;13:651–661. doi: 10.1158/1535-7163.MCT-13-0748. http://dx.doi.org/10.1158/1535-7163.MCT-13-0748. [DOI] [PubMed] [Google Scholar]
- 44.Gee MS, Upadhyay R, Bergquist H, Alencar H, Reynolds F, Maricevich M, Weissleder R, Josephson L, Mahmood U. Human breast cancer tumor models: molecular imaging of drug susceptibility and dosing during HER2/neu-targeted therapy. Radiology. 2008;248:925–935. doi: 10.1148/radiol.2482071496. http://dx.doi.org/10.1148/radiol.2482071496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Myrberg H, Zhang L, Mae M, Langel U. Design of a tumor-homing cell-penetrating peptide. Bioconjug. Chem. 2008;19:70–75. doi: 10.1021/bc0701139. http://dx.doi.org/10.1021/bc0701139. [DOI] [PubMed] [Google Scholar]
- 46.Jiang T, Olson ES, Nguyen QT, Roy M, Jennings PA, Tsien RY. Tumor imaging by means of proteolytic activation of cell-penetrating peptides. Proc. Natl. Acad. Sci. U. S. A. 2004;101:17867–17872. doi: 10.1073/pnas.0408191101. http://dx.doi.org/10.1073/pnas.0408191101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weinstain R, Savariar EN, Felsen CN, Tsien RY. In vivo targeting of hydrogen peroxide by activatable cell-penetrating peptides. J. Am. Chem. Soc. 2014;136:874–877. doi: 10.1021/ja411547j. http://dx.doi.org/10.1021/ja411547j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crisp JL, Savariar EN, Glasgow HL, Ellies LG, Whitney MA, Tsien RY. Dual targeting of integrin alphavbeta3 and matrix metalloproteinase-2 for optical imaging of tumors and chemotherapeutic delivery. Mol. Cancer Ther. 2014;13:1514–1525. doi: 10.1158/1535-7163.MCT-13-1067. http://dx.doi.org/10.1158/1535-7163.MCT-13-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ren T, Liu S, Li G, Zhang J, Guo J, Li W, Yang L. Synthesis, spectroscopic properties and theoretical studies of bis-Schiff bases derived from polyamine and pyrazolones. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012;97:167–175. doi: 10.1016/j.saa.2012.06.005. http://dx.doi.org/10.1016/j.saa.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 50.Agemy L, Friedmann-Morvinski D, Kotamraju VR, Roth L, Sugahara KN, Girard OM, Mattrey RF, Verma IM, Ruoslahti E. Targeted nanoparticle enhanced proapoptotic peptide as potential therapy for glioblastoma. Proc. Natl. Acad. Sci. U. S. A. 2011;108:17450–17455. doi: 10.1073/pnas.1114518108. http://dx.doi.org/10.1073/pnas.1114518108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Primeau AJ, Rendon A, Hedley D, Lilge L, Tannock IF. The distribution of the anticancer drug doxorubicin in relation to blood vessels in solid tumors. Clin. Cancer Res. 2005;11:8782–8788. doi: 10.1158/1078-0432.CCR-05-1664. http://dx.doi.org/10.1158/1078-0432.CCR-05-1664. [DOI] [PubMed] [Google Scholar]
- 52.Heldin CH, Rubin K, Pietras K, Ostman A. High interstitial fluid pressure — an obstacle in cancer therapy. Nat. Rev. Cancer. 2004;4:806–813. doi: 10.1038/nrc1456. http://dx.doi.org/10.1038/nrc1456. [DOI] [PubMed] [Google Scholar]
- 53.Jain RK, Baxter LT. Mechanisms of heterogeneous distribution of monoclonal antibodies and other macromolecules in tumors: significance of elevated interstitial pressure. Cancer Res. 1988;48:7022–7032. [PubMed] [Google Scholar]
- 54.Hu CM, Zhang L. Therapeutic nanoparticles to combat cancer drug resistance. Curr. Drug Metab. 2009;10:836–841. doi: 10.2174/138920009790274540. [DOI] [PubMed] [Google Scholar]
- 55.Huwyler J, Cerletti A, Fricker G, Eberle AN, Drewe J. By-passing of P-glycoprotein using immunoliposomes. J. Drug Target. 2002;10:73–79. doi: 10.1080/10611860290007559. http://dx.doi.org/10.1080/10611860290007559. [DOI] [PubMed] [Google Scholar]
- 56.Hussain S, Rodriguez-Fernandez M, Braun GB, Doyle FJ, 3rd, Ruoslahti E. Quantity and accessibility for specific targeting of receptors in tumours. Sci. Rep. 2014;4:5232. doi: 10.1038/srep05232. http://dx.doi.org/10.1038/srep05232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hamilton AM, Aidoudi-Ahmed S, Sharma S, Kotamraju VR, Foster PJ, Sugahara KN, Ruoslahti E, Rutt BK. Nanoparticles coated with the tumor-penetrating peptide iRGD reduce experimental breast cancer metastasis in the brain. J. Mol. Med. 2015;93:991–1001. doi: 10.1007/s00109-015-1279-x. http://dx.doi.org/10.1007/s00109-015-1279-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen R, Braun GB, Luo X, Sugahara KN, Teesalu T, Ruoslahti E. Application of a proapoptotic peptide to intratumorally spreading cancer therapy. Cancer Res. 2013;73:1352–1361. doi: 10.1158/0008-5472.CAN-12-1979. http://dx.doi.org/10.1158/0008-5472.CAN-12-1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sugahara KN, Scodeller P, Braun GB, deMendoza TH, Yamazaki CM, Kluger MD, Kitayama J, Alvarez E, Howell SB, Teesalu T, Ruoslahti E, Lowy AM. A tumor-penetrating peptide enhances circulation-independent targeting of peritoneal carcinomatosis. J. Control. Release. 2015;212:59–69. doi: 10.1016/j.jconrel.2015.06.009. http://dx.doi.org/10.1016/j.jconrel.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weis SM, Cheresh DA. alphaV integrins in angiogenesis and cancer. Cold Spring Harb. Perspect. Med. 2011;1:a006478. doi: 10.1101/cshperspect.a006478. http://dx.doi.org/10.1101/cshperspect.a006478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yan Z, Zhan C, Wen Z, Feng L, Wang F, Liu Y, Yang X, Dong Q, Liu M, Lu W. LyP-1-conjugated doxorubicin-loaded liposomes suppress lymphatic metastasis by inhibiting lymph node metastases and destroying tumor lymphatics. Nanotechnology. 2011;22:415103. doi: 10.1088/0957-4484/22/41/415103. http://dx.doi.org/10.1088/0957-4484/22/41/415103. [DOI] [PubMed] [Google Scholar]
- 62.Zhang F, Niu G, Lin X, Jacobson O, Ma Y, Eden HS, He Y, Lu G, Chen X. Imaging tumor-induced sentinel lymph node lymphangiogenesis with LyP-1 peptide. Amino Acids. 2012;42:2343–2351. doi: 10.1007/s00726-011-0976-1. http://dx.doi.org/10.1007/s00726-011-0976-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chaudhary A, Hilton MB, Seaman S, Haines DC, Stevenson S, Lemotte PK, Tschantz WR, Zhang XM, Saha S, Fleming T, St Croix B. TEM8/ANTXR1 blockade inhibits pathological angiogenesis and potentiates tumoricidal responses against multiple cancer types. Cancer Cell. 2012;21:212–226. doi: 10.1016/j.ccr.2012.01.004. http://dx.doi.org/10.1016/j.ccr.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hamzah J, Kotamraju VR, Seo JW, Agemy L, Fogal V, Mahakian LM, Peters D, Roth L, Gagnon MK, Ferrara KW, Ruoslahti E. Specific penetration and accumulation of a homing peptide within atherosclerotic plaques of apolipoprotein E-deficient mice. Proc. Natl. Acad. Sci. U. S. A. 2011;108:7154–7159. doi: 10.1073/pnas.1104540108. http://dx.doi.org/10.1073/pnas.1104540108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Uchida M, Kosuge H, Terashima M, Willits DA, Liepold LO, Young MJ, McConnell MV, Douglas T. Protein cage nanoparticles bearing the LyP-1 peptide for enhanced imaging of macrophage-rich vascular lesions. ACS Nano. 2011;5:2493–2502. doi: 10.1021/nn102863y. http://dx.doi.org/10.1021/nn102863y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Greish K. Enhanced permeability and retention (EPR) effect for anticancer nanomedicine drug targeting. Methods Mol. Biol. 2010;624:25–37. doi: 10.1007/978-1-60761-609-2_3. http://dx.doi.org/10.1007/978-1-60761-609-2_3. [DOI] [PubMed] [Google Scholar]
- 67.Christian S, Pilch J, Akerman ME, Porkka K, Laakkonen P, Ruoslahti E. Nucleolin expressed at the cell surface is a marker of endothelial cells in angiogenic blood vessels. J. Cell Biol. 2003;163:871–878. doi: 10.1083/jcb.200304132. http://dx.doi.org/10.1083/jcb.200304132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weissleder R, Kelly K, Sun EY, Shtatland T, Josephson L. Cell-specific targeting of nanoparticles by multivalent attachment of small molecules. Nat. Biotechnol. 2005;23:1418–1423. doi: 10.1038/nbt1159. http://dx.doi.org/10.1038/nbt1159. [DOI] [PubMed] [Google Scholar]
- 69.Tong R, Langer R. Nanomedicines targeting the tumor microenvironment. Cancer J. 2015;21:314–321. doi: 10.1097/PPO.0000000000000123. http://dx.doi.org/10.1097/PPO.0000000000000123. [DOI] [PubMed] [Google Scholar]
- 70.Sugahara KN, Braun GB, de Mendoza TH, Kotamraju VR, French RP, Lowy AM, Teesalu T, Ruoslahti E. Tumor-penetrating iRGD peptide inhibits metastasis. Mol. Cancer Ther. 2015;14:120–128. doi: 10.1158/1535-7163.MCT-14-0366. http://dx.doi.org/10.1158/1535-7163.MCT-14-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kandela I, Chou J, Chow K. Reproducibility Project: Cancer Biology, Reproducibility Project Cancer Biology, Registered report: coadministration of a tumor-penetrating peptide enhances the efficacy of cancer drugs. eLife. 2015;4 doi: 10.7554/eLife.09169. http://dx.doi.org/10.7554/eLife.06959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Song W, Li M, Tang Z, Li Q, Yang Y, Liu H, Duan T, Hong H, Chen X. Methoxypoly(ethylene glycol)-block-poly(l-glutamic acid)-loaded cisplatin and a combination with iRGD for the treatment of non-small-cell lung cancers. Macromol. Biosci. 2012;12:1514–1523. doi: 10.1002/mabi.201200145. http://dx.doi.org/10.1002/mabi.201200145. [DOI] [PubMed] [Google Scholar]
- 73.Zhang Q, Zhang Y, Li K, Wang H, Li H, Zheng J. A novel strategy to improve the therapeutic efficacy of gemcitabine for non-small cell lung cancer by the tumor-penetrating peptide iRGD. PLoS One. 2015;10:e0129865. doi: 10.1371/journal.pone.0129865. http://dx.doi.org/10.1371/journal.pone.0129865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cun X, Chen J, Ruan S, Zhang L, Wan J, He Q, Gao H. A novel strategy through combining iRGD peptide with tumor-microenvironment-responsive and multistage nanoparticles for deep tumor penetration. ACS Appl. Mater. Interfaces. 2015;7:27458–27466. doi: 10.1021/acsami.5b09391. http://dx.doi.org/10.1021/acsami.5b09391. [DOI] [PubMed] [Google Scholar]
- 75.Miao D, Jiang M, Liu Z, Gu G, Hu Q, Kang T, Song Q, Yao L, Li W, Gao X, Sun M, Chen J. Co-administration of dual-targeting nanoparticles with penetration enhancement peptide for antiglioblastoma therapy. Mol. Pharm. 2014;11:90–101. doi: 10.1021/mp400189j. http://dx.doi.org/10.1021/mp400189j. [DOI] [PubMed] [Google Scholar]
- 76.Hu Q, Gu G, Liu Z, Jiang M, Kang T, Miao D, Tu Y, Pang Z, Song Q, Yao L, Xia H, Chen H, Jiang X, Gao X, Chen J. F3 peptide-functionalized PEG–PLA nanoparticles co-administrated with tLyp-1 peptide for anti-glioma drug delivery. Biomaterials. 2013;34:1135–1145. doi: 10.1016/j.biomaterials.2012.10.048. http://dx.doi.org/10.1016/j.biomaterials.2012.10.048. [DOI] [PubMed] [Google Scholar]
- 77.Zhu Z, Xie C, Liu Q, Zhen X, Zheng X, Wu W, Li R, Ding Y, Jiang X, Liu B. The effect of hydrophilic chain length and iRGD on drug delivery from poly(epsiloncaprolactone)-poly(N-vinylpyrrolidone) nanoparticles. Biomaterials. 2011;32:9525–9535. doi: 10.1016/j.biomaterials.2011.08.072. http://dx.doi.org/10.1016/j.biomaterials.2011.08.072. [DOI] [PubMed] [Google Scholar]
- 78.Lao X, Liu M, Chen J, Zheng H. A tumor-penetrating peptide modification enhances the antitumor activity of thymosin alpha 1. PLoS One. 2013;8:e72242. doi: 10.1371/journal.pone.0072242. http://dx.doi.org/10.1371/journal.pone.0072242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang X, Zhen X, Wang J, Zhang J, Wu W, Jiang X. Doxorubicin delivery to 3D multicellular spheroids and tumors based on boronic acid-rich chitosan nanoparticles. Biomaterials. 2013;34:4667–4679. doi: 10.1016/j.biomaterials.2013.03.008. http://dx.doi.org/10.1016/j.biomaterials.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 80.Yu KF, Zhang WQ, Luo LM, Song P, Li D, Du R, Ren W, Huang D, Lu WL, Zhang X, Zhang Q. The antitumor activity of a doxorubicin loaded, iRGD-modified sterically-stabilized liposome on B16-F10 melanoma cells: in vitro and in vivo evaluation. Int. J. Nanomedicine. 2013;8:2473–2485. doi: 10.2147/IJN.S46962. http://dx.doi.org/10.2147/IJN.S46962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu Y, Ji M, Wong MK, Joo KI, Wang P. Enhanced therapeutic efficacy of iRGD-conjugated crosslinked multilayer liposomes for drug delivery. BioMed Res. Int. 2013;2013:378380. doi: 10.1155/2013/378380. http://dx.doi.org/10.1155/2013/378380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gu G, Gao X, Hu Q, Kang T, Liu Z, Jiang M, Miao D, Song Q, Yao L, Tu Y, Pang Z, Chen H, Jiang X, Chen J. The influence of the penetrating peptide iRGD on the effect of paclitaxel-loaded MT1-AF7p-conjugated nanoparticles on glioma cells. Biomaterials. 2013;34:5138–5148. doi: 10.1016/j.biomaterials.2013.03.036. http://dx.doi.org/10.1016/j.biomaterials.2013.03.036. [DOI] [PubMed] [Google Scholar]
- 83.Puig-Saus C, Rojas LA, Laborda E, Figueras A, Alba R, Fillat C, Alemany R. iRGD tumor-penetrating peptide-modified oncolytic adenovirus shows enhanced tumor transduction, intratumoral dissemination and antitumor efficacy. Gene Ther. 2014;21:767–774. doi: 10.1038/gt.2014.52. http://dx.doi.org/10.1038/gt.2014.52. [DOI] [PubMed] [Google Scholar]
- 84.Du R, Zhong T, Zhang WQ, Song P, Song WD, Zhao Y, Wang C, Tang YQ, Zhang X, Zhang Q. Antitumor effect of iRGD-modified liposomes containing conjugated linoleic acid-paclitaxel (CLA-PTX) on B16-F10 melanoma. Int. J. Nanomedicine. 2014;9:3091–3105. doi: 10.2147/IJN.S65664. http://dx.doi.org/10.2147/IJN.S65664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shen J, Meng Q, Sui H, Yin Q, Zhang Z, Yu H, Li Y. iRGD conjugated TPGS mediates codelivery of paclitaxel and survivin shRNA for the reversal of lung cancer resistance. Mol. Pharm. 2014;11:2579–2591. doi: 10.1021/mp400576f. http://dx.doi.org/10.1021/mp400576f. [DOI] [PubMed] [Google Scholar]
- 86.Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ, Wei J, Nie G. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35:2383–2390. doi: 10.1016/j.biomaterials.2013.11.083. http://dx.doi.org/10.1016/j.biomaterials.2013.11.083. [DOI] [PubMed] [Google Scholar]
- 87.De G, Ko JK, Tan T, Zhu H, Li H, Ma J. Amphipathic tail-anchoring peptide is a promising therapeutic agent for prostate cancer treatment. Oncotarget. 2014;5:7734–7747. doi: 10.18632/oncotarget.2301. http://dx.doi.org/10.18632/oncotarget.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mao X, Liu J, Gong Z, Zhang H, Lu Y, Zou H, Yu Y, Chen Y, Sun Z, Li W, Li B, Gao J, Zhong Y. iRGD-conjugated DSPE-PEG2000 nanomicelles for targeted delivery of salinomycin for treatment of both liver cancer cells and cancer stem cells. Nanomedicine. 2015;10:2677–2695. doi: 10.2217/nnm.15.106. http://dx.doi.org/10.2217/nnm.15.106. [DOI] [PubMed] [Google Scholar]
- 89.Yan F, Wu H, Liu H, Deng Z, Liu H, Duan W, Liu X, Zheng H. Molecular imaging-guided photothermal/photodynamic therapy against tumor by iRGD-modified indocyanine green nanoparticles. J. Control. Release. 2015;224:217–228. doi: 10.1016/j.jconrel.2015.12.050. http://dx.doi.org/10.1016/j.jconrel.2015.12.050. [DOI] [PubMed] [Google Scholar]
- 90.Dai W, Fan Y, Zhang H, Wang X, Zhang Q, Wang X. A comprehensive study of iRGD-modified liposomes with improved chemotherapeutic efficacy on B16 melanoma. Drug Deliv. 2015;22:10–20. doi: 10.3109/10717544.2014.903580. http://dx.doi.org/10.3109/10717544.2014.903580. [DOI] [PubMed] [Google Scholar]
- 91.Ni D, Ding H, Liu S, Yue H, Bao Y, Wang Z, Su Z, Wei W, Ma G. Superior intratumoral penetration of paclitaxel nanodots strengthens tumor restriction and metastasis prevention. Small. 2015;11:2518–2526. doi: 10.1002/smll.201403632. http://dx.doi.org/10.1002/smll.201403632. [DOI] [PubMed] [Google Scholar]
- 92.Li M, Tang Z, Zhang D, Sun H, Liu H, Zhang Y, Zhang Y, Chen X. Doxorubicin-loaded polysaccharide nanoparticles suppress the growth of murine colorectal carcinoma and inhibit the metastasis of murine mammary carcinoma in rodent models. Biomaterials. 2015;51:161–172. doi: 10.1016/j.biomaterials.2015.02.002. http://dx.doi.org/10.1016/j.biomaterials.2015.02.002. [DOI] [PubMed] [Google Scholar]