Fig. 1.
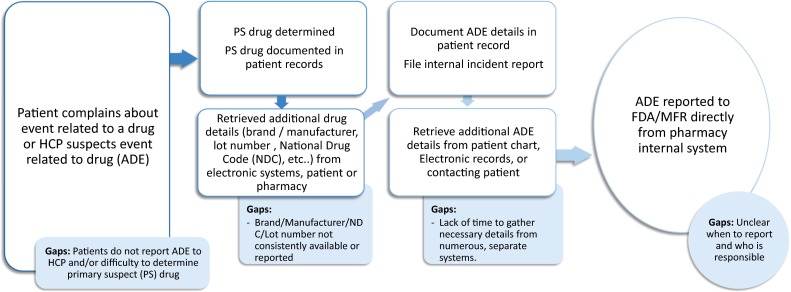
Process flow and main gaps identified for adverse drug event reporting across all settings. ADE adverse drug event, HCP healthcare provider, MFR manufacturer, NDC National Drug Code, PS primary suspect

Process flow and main gaps identified for adverse drug event reporting across all settings. ADE adverse drug event, HCP healthcare provider, MFR manufacturer, NDC National Drug Code, PS primary suspect