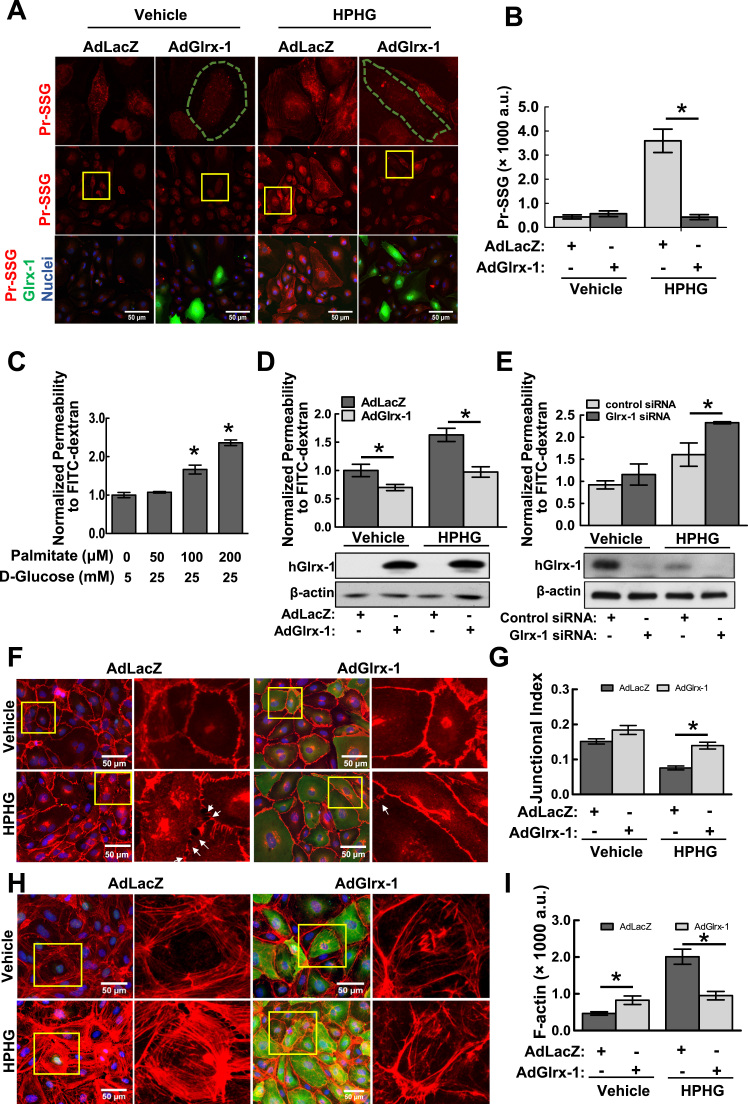
Fig. 2.
Adenoviral overexpression of Glrx-1 attenuates metabolic stress-induced protein S-glutathionylation and endothelial cell permeability. A–B: Overexpression of Glrx-1 abrogates HPHG-induced PrS-SG in ECs. HAECs were infected with an adenovirus expressing human Glrx-1 or a control adenovirus (AdlacZ) for 24 h. Cells were then treated with vehicle (25 μM BSA, 5 mM glucose, 20 mannitol) and HPHG (100 μM palmitate-BSA conjugate, 25 mM glucose) in a serum-free medium for another 24 h, followed by immunostaining for PrS-SG (red channel) and Glrx-1 (green channel) with antibodies against GSH and Glrx-1, respectively. The first row shows high magnification views of yellow boxed areas. Green outlined cells overexpress Glrx-1. Fifteen randomly selected image fields from three independent experiments were used for quantification (n=15). *p≤0.05 between indicated groups. C: HPHG treatment increases endothelial cell permeability in a dose-dependent manner. HAEC monolayers cultured onto Transwell inserts were exposed to HPHG at indicated concentrations for 2 h, followed by FITC-dextran influx assay. Results are the normalized percentages of total FITC-dextran passing across monolayers relative to the control group and represented as mean ± SD of three independent experiments, each performed in triplicates. *p<0.05, compared with vehicle group. D-E: Adenoviral overexpression of Glrx-1 (D) protected against, siRNA-mediated downregulation of Glrx-1(E) exacerbated HPHG-induced permeability. The expression of Glrx-1 in HAECs infected with adenovirus or siRNAs were assessed by immunoblotting shown in the lower panels of D and E, respectively. *p<0.05 compared between indicated groups. F–I: Overexpression of Glrx-1 in HAECs protected cell-cell adherens junctions and actin cytoskeletal structures against HPHG challenge. Adenovirus-infected HAECs were exposed to vehicle (50 μM BSA, 5 mM glucose, 20 mM mannitol) or HPHG (200 μM palmitate-BSA conjugate, 25 mM glucose) for 2 h, followed by immunostaining for VE-Cadherin, a marker for cell-cell junctions (F, red channel), and Glrx-1 (F and H, green channel). F-actin was stained with red fluorescence-labeled phalloidin (H). The second and last column shows high magnified views of yellow boxed areas. Junctional index (G) as a measure of barrier integrity (VE-Cadherin staining density/total area of image field)×100/cell number), and F-actin staining intensity (F) were measured in 15 randomly selected image fields from three independent experiments. Intercellular gaps were indicated by white arrows. Results represent mean±SEM (n=15). *p<0.05, compared between indicated groups. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)