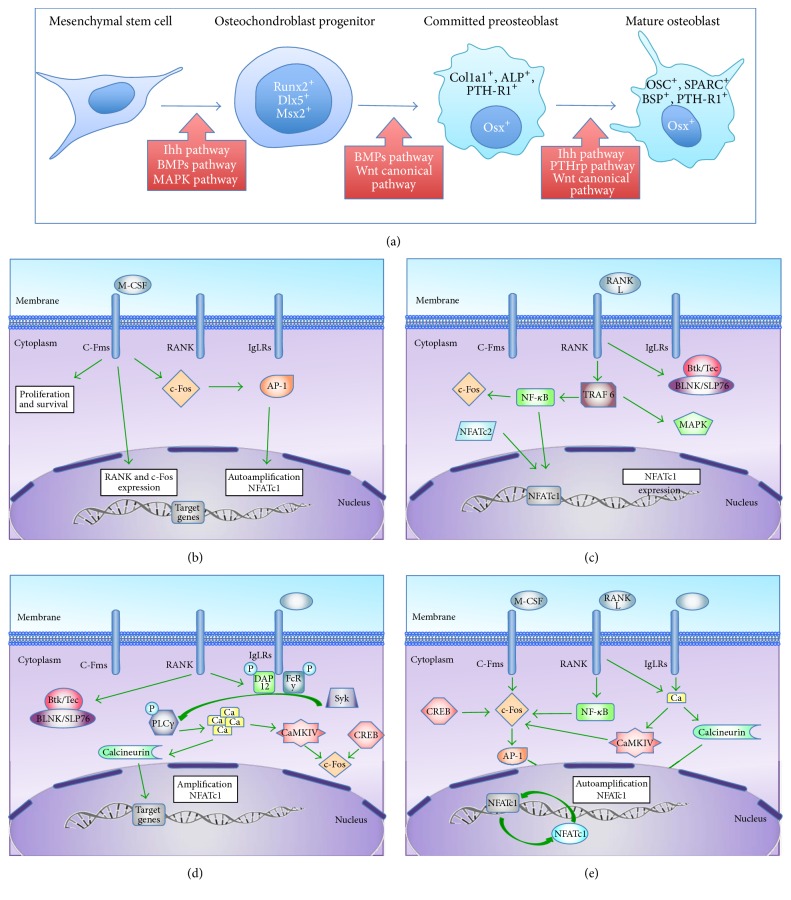
Figure 3.
Bone remodeling: (a) in precursor cell stage, the macrophage-colony stimulating factor (M-CSF) binds to its receptor (c-Fms). It promotes survival and proliferation of osteoclast precursors, as well as RANK and c-Fos expression. (b) c-Fms pathway: M-CSF binds to c-Fms and promotes cell proliferation and survival. It also promotes RANK and c-Fos expression as well as NFATc1 autoamplification through AP1. (c) RANK pathway: the binding of RANKL to RANK promotes the recruitment of tumor necrosis factor receptor-associated factor 6 (TRAF6), which can activate the nuclear factor-κB (NF-κB) and mitogen-activated protein kinases (MAPKs), such as p38 and Jun N terminal kinase (JNK). TRAF6-activated NF-κB induces the expression of NFATc1, an important transcription factor for osteoclastogenesis. This NFATc1 expression is also stimulated by the nuclear factor of activated T cells cytoplasmic 2 (NFATc2). Finally, RANK also activates the tyrosine kinases Btk and Tec that are involved in the phosphorylation of phospholipase Cγ (PLC γ). NF-κB can also stimulate the c-Fos induction. (d) IgLRs pathway and calcium signaling: RANK activation leads to phosphorylation of DAP12 and FcRγ. These molecules are both associated with IgLRs and stimulate Syk. Activated Btk/Tec/BLNK/SLP76 complex and Syk will phosphorylate PLC-γ which will mediate the calcium release from intracellular stores. Calcium will activate calcineurin phosphatase which is involved in NFATC1 autoamplification. It also stimulates C-Fos through CaMKIV activation. (e) NFATc1 autoamplification: by these three pathways, AP-1, calcineurin and NFATc1 participate in NFATc1 autoamplification. Indeed, AP-1 and the continuous calcium signaling are essential for NFATc1 amplification. The NFATc1 promoter is epigenetically activated through histone acetylation and contains NFAT binding sites. Thus, NFATc1 specifically autoregulates its own promoter and is responsible for its robust induction. NFATc1 is negatively regulated by other transcription factors, such as IRF8, MafB, and Bcl6 that are, in turn, inhibited by Blimp1, a transcriptional target of NFATc1.