Figure 1.
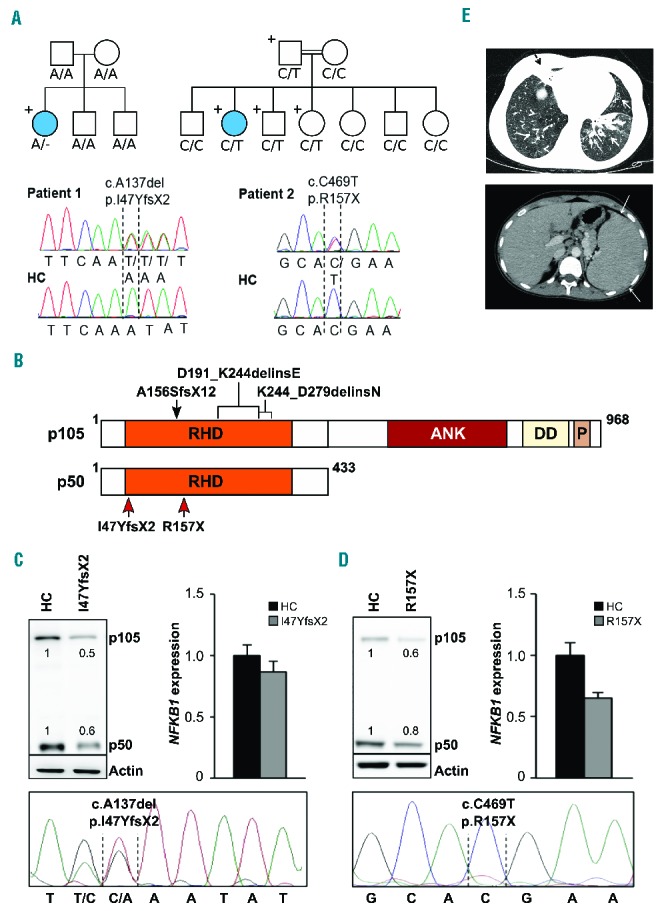
Two novel heterozygous NFKB1 mutations detected in two families decrease the protein levels of p105 and p50. (A) Upper left: Whole exome sequencing identified a heterozygous NFKB1 mutation (A/−) in patient 1. The patient is the only carrier of the mutation in the family pedigree (indicated by “+”) and the only diseased family member (indicated by a filled circle). Lower left: Capillary sequencing using genomic DNA confirmed an NFKB1 frameshift mutation (c.A137del, p.I47YfsX2) in patient 1. Representative chromatograms of patient 1 and a healthy control (HC) are shown. Upper right: Patient 2 descended from consanguineous parents and harbors an inherited heterozygous NFKB1 mutation (C/T). The patient is the only diseased family member. The father and two siblings carry the same mutation but are not affected. Sanger sequencing of NFKB1 confirmed the heterozygous missense mutation (c.C469T, p.R157X) in patient 2. Representative chromatograms are shown. (B) Schematic drawing of the proteins p105 and p50 and their domains which are both encoded by the NFKB1 gene. The mutations in the Rel homology domain (RHD) identified in the two patients (red arrows) lead to early truncation of both proteins. Previously reported heterozygous germline mutations associated with CVID are indicated on top (black arrow and brackets). ANK, ankyrin repeats; DD, death domain; P, PEST domain enriched for proline (P), glutamic acid (E), serine (S), and threonine (T) residues. (C, D) Expression of p105 and p50 proteins is decreased in the affected patients. (C) Primary T cells of patient 1 and healthy controls were activated by phytohemagglutinin in the presence of interleukin-2. Protein and RNA extracts were prepared. Western blot analysis was carried out employing a specific p105/p50 antibody using β-actin as a loading control (left panel). NFKB1 mRNA expression was measured by real-time polymerase chain reaction (right panel). The fold-change in cells of the patient compared to a representative healthy control is shown, GAPDH and β-actin expression were used as internal standards. Mean values of representative experiments performed in triplicates and corresponding SDs are shown. Sanger sequencing using reverse transcribed mRNA of the patient demonstrates the presence of mutated NFKB1 transcripts (lower panel). (D) Epstein-Barr virus-transformed B cells of patient 2 were used for protein and RNA extraction. Analysis of NFKB1 protein and RNA expression was carried out as described in (C). Capillary sequencing of cDNA from patient 2 failed to detect the NFKB1 mutation indicating that the mutation leads to mRNA instability (lower panel). (E) Upper panel: axial high resolution chest computer tomography image of patient 2 at the level of lung bases demonstrating multiple areas of bronchiectasis (white arrows) and consolidation with an atelectatic component surrounding bronchiectases in the right middle lobe (black dashed arrow). Mosaic pattern of perfusion of the lung parenchyma is noted, with multiple areas of low attenuation in the right low lobe (arrowheads). Lower panel: axial computer tomography image of patient 2 at the level of the upper abdomen demonstrating the enlarged spleen.
