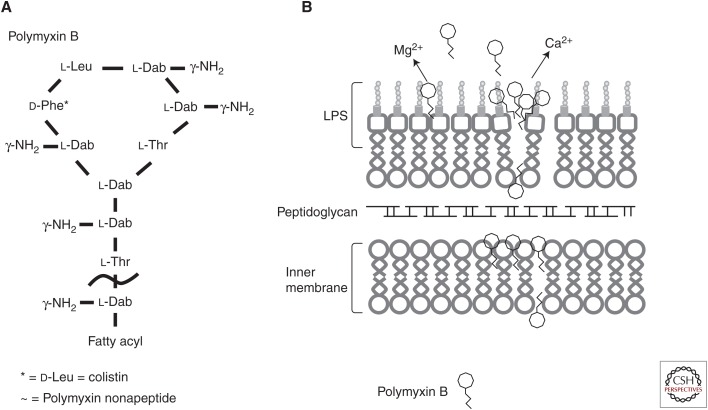
Figure 1.
The structure of polymyxin B and likely mode of membrane interaction. (A) The general structure of the cyclic cationic peptide polymyxin B. Colistin (polymyxin E) replaces the phenylalanine (d-Phe*) in polymyxin B with a leucine. Attenuated polymyxin nonapeptide has the fatty acyl tail removed adjacent to the threonine outside the ring structure (∼). (B) Polymyxin B interacts with the lipid A portion of the lipopolysaccharide (LPS) outer membrane. The peptides cross the outer membrane through a “self-promoted uptake” mechanism and then interact with the cytoplasmic membrane to inhibit cellular energization, and possibly cause inhibition of cell division and/or cytoplasmic membrane permeabilization and subsequent cell death.