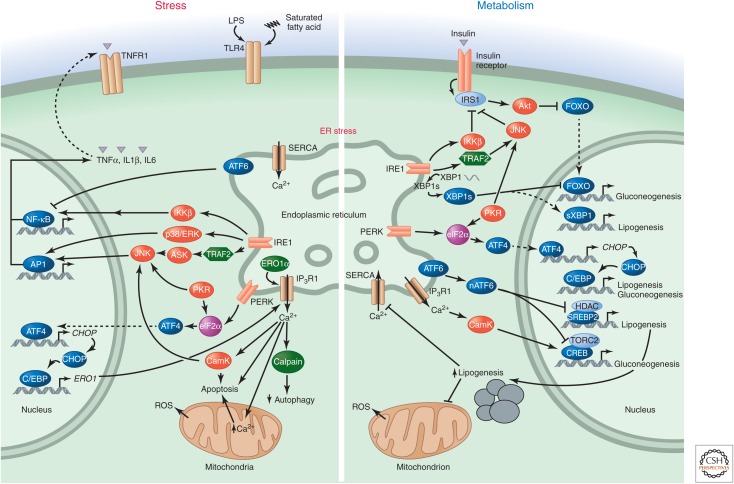
Figure 3.
The UPR in stress signaling, inflammation, and metabolism. The UPR contributes to both inflammatory/stress signaling and metabolic regulation, as exemplified in chronic metabolic diseases. It activates several stress-related kinases including ERK, p38, JNK, and IKK. The resulting signals can impair signaling by insulin or other endocrine hormones and disrupt metabolism. The UPR can also modulate glucose and lipid metabolism directly. Nuclear ATF6 inhibits gluconeogenesis and lipogenesis by directly binding to TORC2 and SREBP2 proteins, respectively. This mechanism is defective in metabolic disease. The spliced form of XBP1 (XBP1s) can directly or indirectly (through SREBP1) activate the lipogenesis program while inhibiting gluconeogenesis. eIF2α phosphorylation leads to the synthesis of CHOP and the activation of lipogenesis programs via CEBPα/β. (Note that the opposing effects of the UPR on gluconeogenesis and lipogenesis are context dependent.) Activation of lipogenesis alters the membrane lipid composition of the ER, inhibits SERCA calcium pumps, and propagates ER stress, which in turn disrupts metabolism further. Hence, inflammatory, stress, and metabolic responses generate a vicious cycle if the ER dysfunction cannot be remedied.