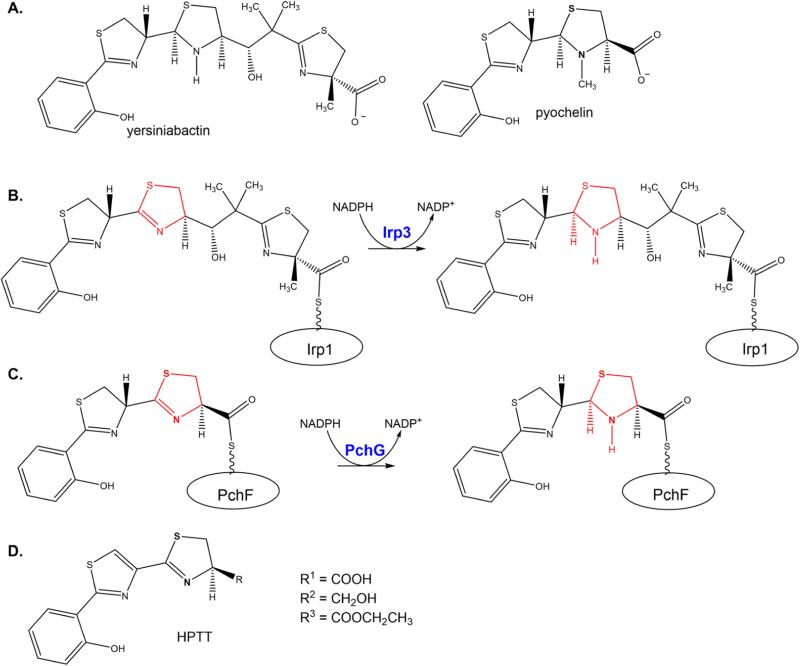
Scheme 1.
Yersiniabactin and Pyochelin and the Required Reductase Activities for Their Biosynthesisa
a(A) Mature yersiniabactin and pyochelin siderophores. The stereochemistry for yersiniabactin and pyochelin was taken from refs 3 and 40—44. (B) Irp3 catalyzes the reduction of the C-terminal thiazoline ring to thiazolidine while the substrate is tethered to the nonribosomal peptide synthetase Irp1 through a post-translational modification that is the basis of the thiotemplate mechanism. (C) Pyochelin, the siderophore produced by P. aeruginosa, is produced in the pathway with the stand-alone reductase PchG. PchG catalyzes the reduction of the same thiazoline ring as Irp3 while the substrate is covalently bound to the nonribosomal peptide synthetase PchF. (D) Substrate analogue HPTT.