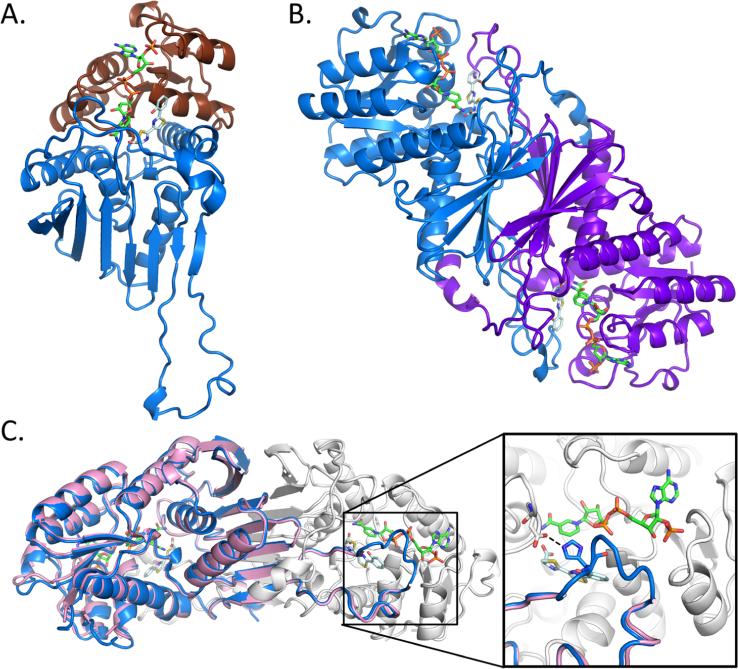
Figure 1.
Overall structure of Irp3. (A) The monomer structure of Irp3 forms two domains, the N-terminal NADP(H) binding domain (chocolate cartoon) and the C-terminal dimerization domain (blue cartoon). (B) Dimeric structure of Irp3 with monomer A colored blue and monomer B colored purple. (C) The new crystal form of Irp3 (blue and white cartoons) allows for M—N loop closure over the active site of the opposing monomer. The M—N loop was disordered in the original crystal form (pink cartoon). The inset shows a close-up of the active site and the binding site of NADP+ (green sticks) and HPTT-COOH (pale cyan sticks). The hydrogen bond between the M—N loop (histidine 257) of one monomer and the active site cavity (aspartic acid 217) of the other monomer is highlighted. Protein structure figures were generated using PyMOL.45