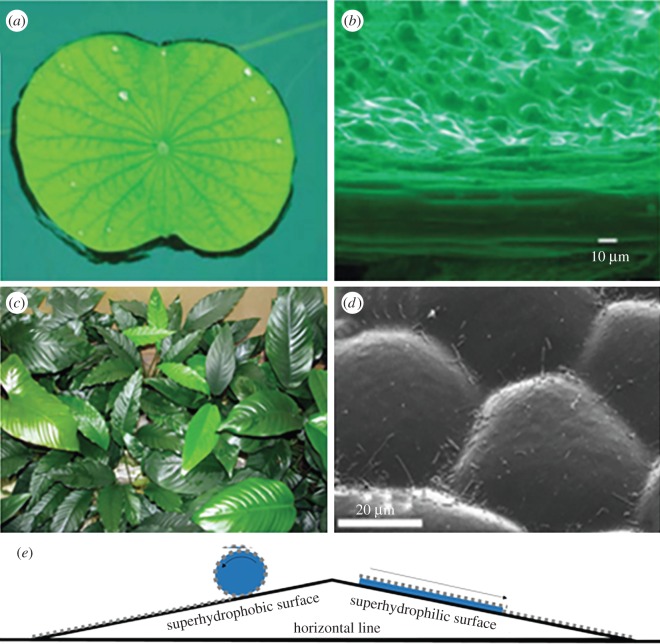
Figure 1.
Natural plants and their surface morphology. (a) Lotus leaf. (Reproduced from [35] with permission of The Royal Society of Chemistry.) (b) Scanning electron microscopy (SEM) picture of lotus leaves. (Reproduced from [35] with permission of The Royal Society of Chemistry.) (c) Anubias leaves. (d) SEM picture of Anubias leaves. (Reproduced from [2] with permission of The Royal Society.) (e) Demonstration of self-cleaning on a superhydrophobic surface and a superhydrophilic surface. For the superhydrophobic case, the particles on the surface can be removed by the rolling off of water droplets due to the high contact angle (usually greater than 150°) between the surface and the droplet. For the superhydrophilic surfaces, the particles on the surface can be easily separated by the small contact angle between the droplet and the surface and then taken away due to the good contact between the surface and the droplet.