Fig. 1.
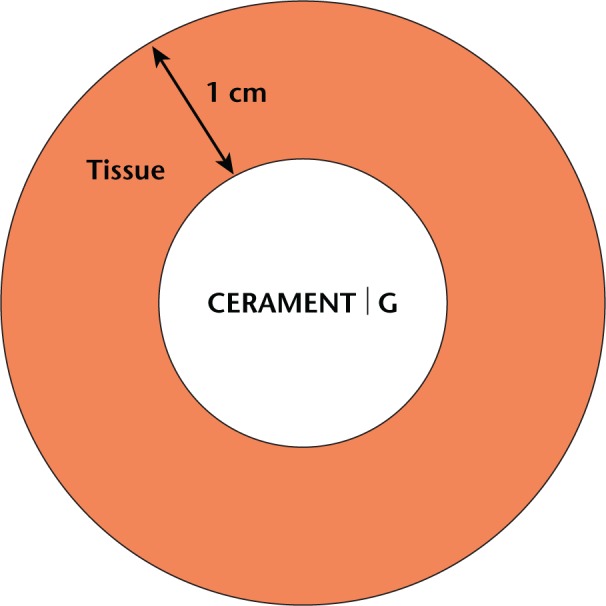
Illustration of the local release model used for the in vitro release test. In an in vivo condition the minimal inhibitory concentration levels for gentamicin-sensitive microorganisms should be reached in all the tissue surrounding the device up to a distance of approximately 1 cm. Since the volume of the implant is 10 mL, the surrounding volume of a distance up to 1.09 cm is approximately 50 mL.
