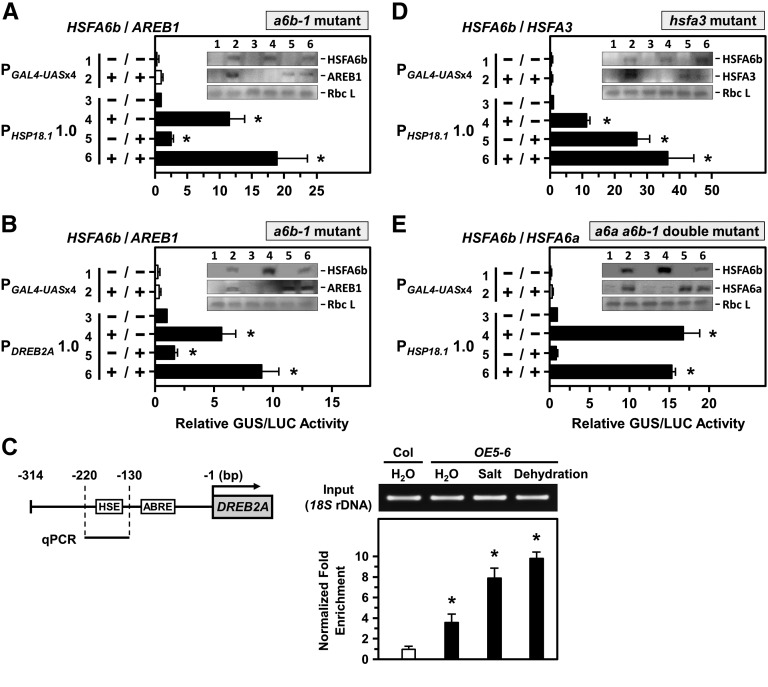
Figure 7.
HSFA6b and AREB1 activated the transcriptional activity of the HSP18.1-CI and DREB2A promoters. A and B, a6b-1 mutant protoplasts were used for a transcriptional activation assay, as indicated in Figure 4. The 1-kb length of the HSP18.1-CI or DREB2A promoter region, respectively, was fused with GUS reporter gene. Protoplasts were transfected with or without effectors HSFA6b-3XFLAG and AREB1-3XFLAG. The effectors were analyzed by immunoblotting (insets). The fold expression was normalized relative to the level of PHSP18.1 and PDREB2A without effectors. C, HSFA6b binding to the HSE sequence of the DREB2A promoter was analyzed by ChIP assay. Schematic map of the 314-bp upstream of the DREB2A promoter region (left); ABRE (GACACGTA; −86 to −93 bp) and HSE (AGAAGATTCG; −151 to −160 bp) are in gray boxes. Seedlings were treated with 6 h 150 mm NaCl or 2.5 h dehydration. The fold enrichment of the HSE-containing region (qPCR) after ChIP was analyzed by qRT-PCR and normalized to the Col H2O treatment (right). 18S rDNA was an input control. D and E, Transcriptional activation assay in hsfa3 and a6a a6b-1 double-mutant protoplasts with or without effectors HSFA6b-3XFLAG, HSFA3-3XFLAG, and HSFA6a-3XFLAG are as described in A and B. The fold expression was normalized relative to that of the PHSP18.1 without effectors. Data are means ± sd of three biological replicates. *Significant at P < 0.05.