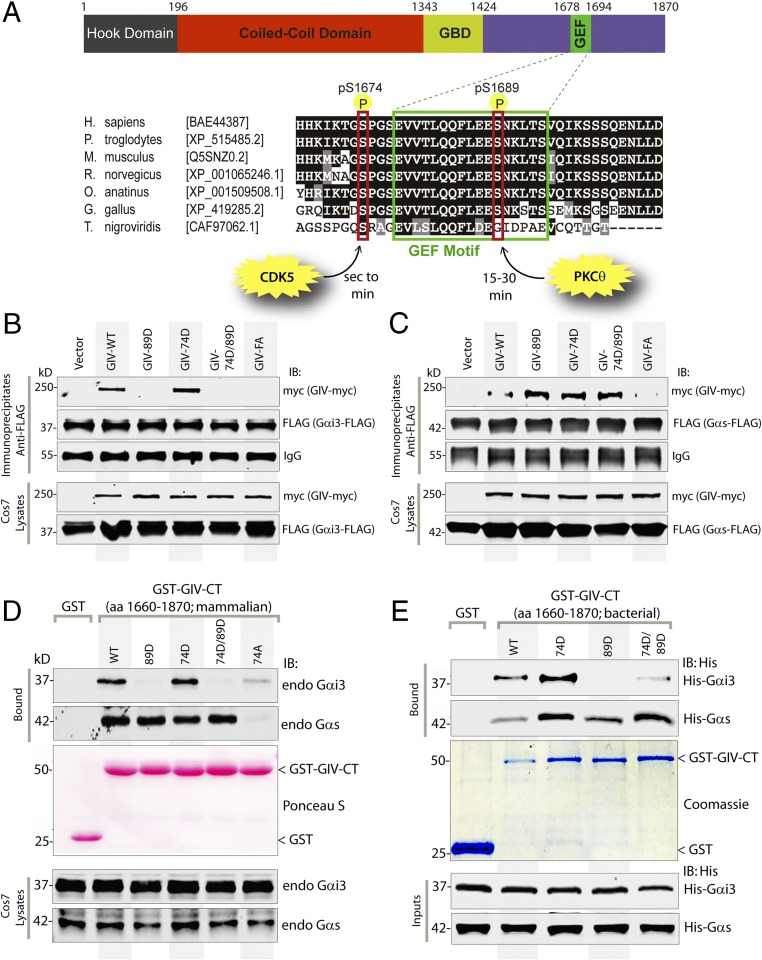
Fig. 2.
Sequential phosphorylation of GIV by CDK5 and PKCθ triggers sequential GIV–Gαi3 and GIV–Gαs interactions. (A) Schematic of the domain architecture of GIV and sequence alignment of its C-terminal GEF motif. (Upper) Various domains of GIV are shown. Residue numbers marking the boundaries of each domain are shown. (Lower) The sequence encompassing the GEF motif (green rectangle) and surrounding residues was aligned among various species (accession numbers are shown in brackets) using ClustalW. Conserved residues are shaded in black, and similar residues are shaded in gray. The two phosphorylated residues (S1674 and S1689 in human GIV) that respectively activate (11) and inactivate (13) the GEF function are boxed in red. The timing of these phosphoevents after EGF stimulation and the kinases responsible for these two regulatory phosphoevents [CDK5 (11) and PKCθ (13)] are shown also. (B and C) Immunoprecipitation was carried out with anti-FLAG antibody on equal aliquots of lysates of Cos7 cells coexpressing Gαi3-FLAG (B) or FLAG-Gαs (C) with full-length Myc-tagged GIV (WT and mutants), followed by incubation with protein-G Sepharose beads. Immunoprecipitates (Upper) and lysates (Lower) were analyzed for GIV-Myc and Gαi3-FLAG (B) or Gαs-FLAG (C) by immunoblotting. (D) Lysates of Cos7 cells expressing WT or mutant GST-GIV-CT were incubated with glutathione-Sepharose beads. Bound proteins (Upper) and lysates (Lower) were analyzed by immunoblotting for endogenous (endo) Gαi3 and Gαs and were quantified using band densitometry (Fig. S3A). Equal loading of GST proteins was confirmed by Ponceau-S staining. (E) Equimolar amounts of purified His-Gαi3 (Upper) or His-Gαs (Lower) were incubated with GST or GST-GIV-CT proteins (WT and mutants) immobilized on glutathione-Sepharose beads (Coomassie). Bound His-Gαi3 or His-Gαs was analyzed by immunoblotting using anti-His mAb and was quantified using band densitometry (Fig. S3B). Equal loading of inputs was confirmed by immunoblotting using anti-His mAb.