Significance
Studies on the importance of vitamin A for human health continue to draw significant worldwide attention. However, the instability of provitamin A in crops resulted in a significant reduction of the potential nutrition values of these food crops. Our work demonstrates that provitamin A can be stabilized in sorghum by the coexpression of vitamin E through ectopic expression of homogentisate geranylgeranyltransferase (HGGT) and that vitamin E can enhance the stability of provitamin A in planta. This research has the potential to impact directly the lives of the millions of people who suffer from vitamin A deficiency, and we believe that these results will be applicable to enhancing provitamin A stability in many food crops.
Keywords: β-carotene accumulation, β-carotene stability, vitamin E, HGGT, biofortified sorghum
Abstract
Micronutrient deficiencies are common in locales where people must rely upon sorghum as their staple diet. Sorghum grain is seriously deficient in provitamin A (β-carotene) and in the bioavailability of iron and zinc. Biofortification is a process to improve crops for one or more micronutrient deficiencies. We have developed sorghum with increased β-carotene accumulation that will alleviate vitamin A deficiency among people who rely on sorghum as their dietary staple. However, subsequent β-carotene instability during storage negatively affects the full utilization of this essential micronutrient. We determined that oxidation is the main factor causing β-carotene degradation under ambient conditions. We further demonstrated that coexpression of homogentisate geranylgeranyl transferase (HGGT), stacked with carotenoid biosynthesis genes, can mitigate β-carotene oxidative degradation, resulting in increased β-carotene accumulation and stability. A kinetic study of β-carotene degradation showed that the half-life of β-carotene is extended from less than 4 wk to 10 wk on average with HGGT coexpression.
The importance of vitamin A for human health has been widely addressed (1–6). A 2009 Global Report (7) summarized vitamin A as being “vital for survival and sight; to boost the immune system, vitamin A is a critical micronutrient for survival and physical health of children exposed to disease.” In Africa, malnutrition is a serious challenge, but micronutrient deficiency also plays a dominant role in the overall food security of that continent. Based on this global report, the five countries having the highest proportions of preschool age children with vitamin A deficiency were all located in Africa: 95.6% in Sao Tome and Principe, 84.4% in Kenya, 75.8% in Ghana, 74.8% in Sierra Leone, and 68.8% in Mozambique.
Sorghum (Sorghum bicolor L.) is one of the most important staple foods for an estimated 500 million people, primarily those living in arid and semiarid areas. In Africa, it is the second most important cereal; about 300 million people rely on it as their daily staple food. Although sorghum is gluten-free and could be an attractive replacement for wheat-allergy sufferers, it is considered a nutrient-poor crop (8, 9) with very low amounts of β-carotene (10). The improvement of micronutrients in food crops has attracted considerable attention, and significant advances have been made in a range of major crops (11–22). Nutritional improvement in sorghum was undertaken a decade ago (23, 24); however, progress has lagged behind the progress in other crops. One reason was the recalcitrance of sorghum to genetic modification via transformation. Recent improvements in sorghum transformation have largely overcome this barrier and offer an alternative approach to genetic improvements in sorghum (25).
One of our objectives is to develop sorghum lines with enhanced and stabilized provitamin A (β-carotene). In Africa sorghum grain is commonly stored in ambient conditions for several months between harvest and consumption. The stability of β-carotene during storage is important for maintaining its nutritional value. During this study, a challenging issue was ensuring the stability during storage of the β-carotenes that were enhanced in our transgenic sorghum grain. A series of experiments revealed that oxidation was the major factor causing β-carotene degradation in sorghum grain. We found that coexpressing barley homogentisate geranylgeranyltransferase (HGGT) (26, 27) with genes responsible for enhancing β-carotene levels significantly improved β-carotene accumulation and stability in sorghum grain.
Results
Enhancing All-Trans β-Carotene Accumulation in Sorghum Endosperm.
Sorghum contains very low levels of β-carotene. Approximately 45 d after pollination (DAP), mature WT TX430 inbred seeds accumulate 0.5 µg/g dry weight (DW) all-trans β-carotene and moderate levels of lutein (2.04 µg/g) and zeaxanthin (2.57 µg/g) (Fig. S1 and Table S1), which could correlate with the light yellow color of TX430 seeds (10). Because all-trans β-carotene was the predominant provitamin A carotenoid in both transgenic and WT sorghum grains (Fig. S1 and Table S1), we focus only on all-trans β-carotene in this report. To increase β-carotene accumulation in sorghum endosperm, we first tested the genes described by the Golden Rice-2 (GR2) research team (28) in sorghum and constructed vector–ABS168 containing the Zea mays phytoene synthase 1 (PSY1) and the Pantoea ananatis carotene desaturase (CRTI) genes driven with different sorghum endosperm-specific promoters as illustrated in Fig. 1A. However, implementing the technology developed for GR2 in sorghum achieved limited improvement in all-trans β-carotene accumulation, perhaps because of the different crop species and/or different promoters that drive PSY1 and CRTI genes. All transgenic ABS168 quality events (see Materials and Methods for the definition of a “quality event”), such as event ABS168-1, accumulated less than 2 µg/g DW all-trans β-carotene in the mature seeds, without a visible change in seed color compared with WT (Fig. 2A). The events bearing multiple copies of the genes accumulated higher levels of total carotenoids in mature seeds, as previously reported (29). To investigate carotenoid accumulation during seed development, we further examined changes in seed color at different stages of seed developmental using a multiple-copy event ABS168-A. A light yellow color developed in the immature ABS168-A seeds around 30 DAP (Fig. 2B). This observation suggested that higher levels of carotenoids were produced in an early stage of seed development and that the decoloration occurred during seed maturation, further suggesting that the final carotenoid content in the seeds could reflect the balance between their biosynthesis vs. in planta degradation. This observation is consistent with a previous study demonstrating that carotenoid content increased at an early stage of kernel development and decreased sharply as kernels approached maturity in nontransgenic sorghum seeds (10).
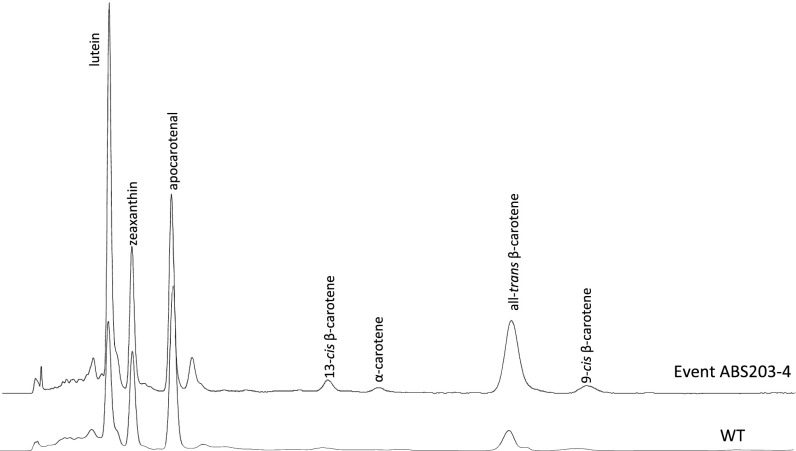
Fig. S1.
Representative HPLC chromatograms of mature WT and event ABS203-4 seeds. The carotenoid composition levels are listed in Table S1.
Table S1.
Carotenoid profiles of mature WT and event ABS203-4 seeds
| Lutein, μg/g | Zeaxanthin, μg/g | 13-Cis β-carotene, μg/g | α-Carotene, μg/g | All-trans β-carotene, μg/g | 9-Cis β-carotene, μg/g | |
| WT | 2.04 | 2.57 | 0.22 | ND | 0.50 | 0.23 |
| Event ABS203-4 | 9.50 | 5.15 | 1.39 | 1.08 | 12.30 | 1.20 |
| Fold change ABS203-4/WT | 4.70 | 2.00 | 6.12 | ND | 24.6 | 2.21 |
Data represent the HPLC data in Fig. S1.
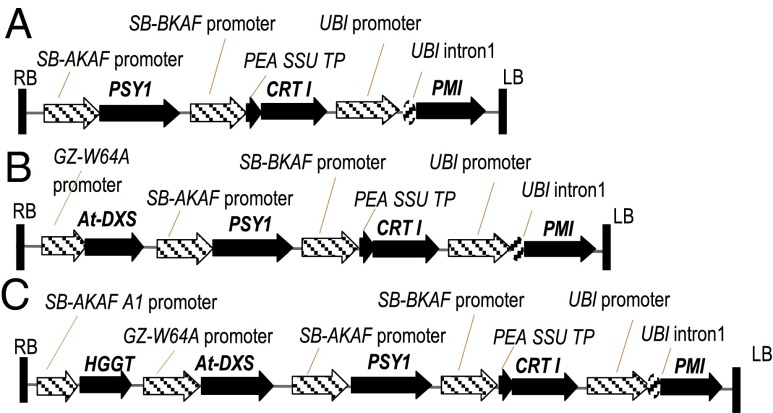
Fig. 1.
Schematic representation of the molecular components in the transfer DNA (T-DNA) regions of the binary vectors used in sorghum transformations (see SI Materials and Methods for details). (A) Vector–ABS168. (B) Vector–ABS198. (C) Vector–ABS203.
Fig. 2.
Comparison of seed color in WT and transgenic sorghum. (A) Color in mature WT, single-copy event ABS168-1, ABS198-1, and ABS203-4 seeds. (B) Color in the endosperm of immature WT and event ABS168-A (multiple-copy event) seeds at 30 DAP. (C) Color of the endosperm of mature WT and ABS203-4 seeds.
DXS (1-deoxyxylulose 5-phosphate synthase) controls the rate-limiting step of the methylerythritol phosphate pathway that provides the precursor for carotenoid biosynthesis. It has been reported that overexpression of Arabidopsis At-DXS facilitates carotenoid biosynthesis in planta (30, 31). To improve carotenoid synthesis in sorghum further, vector–ABS198 was designed in which At-DXS was coexpressed with PSY1 and CRTI (Fig.1B). The top five ABS198 quality events accumulated higher levels of total carotenoids (Table S2), and the all-trans β-carotene levels ranged from 2.5 to 9.1 µg/g DW in the mature seeds (Table 1). The endosperm of the best-performing ABS198 quality event, ABS198-1, accumulated all-trans β-carotene up to 9.1 µg/g DW and showed a slightly visible orange color in the mature seeds (Fig. 2A).
Table S2.
Major carotenoid profiles of mature event ABS203 and event ABS198 seeds
| Event | Lutein, μg/g | Zeaxanthin, μg/g | All-trans β-carotene, μg/g |
| ABS203-1 | 9.32 ± 0.26 | 4.04 ± 0.16 | 7.31 ± 0.65 |
| ABS203-2 | 10.51 ± 0.52 | 5.14 ± 0.19 | 10.49 ± 0.85 |
| ABS203-3 | 11.46 ± 0.12 | 5.73 ± 0.08 | 8.51 ± 0.92 |
| ABS203-4 | 10.54 ± 0.44 | 5.17 ± 0.29 | 12.30 ± 1.43 |
| ABS203-5 | 10.48 ± 0.16 | 5.95 ± 0.14 | 7.91 ± 0.51 |
| ABS198-1 | 12.01 ± 0.36 | 5.34 ± 0.04 | 9.06 ± 1.31 |
| ABS198-2 | 9.60 ± 0.16 | 5.43 ± 0.6 | 5.87 ± 0.37 |
| ABS198-3 | 6.94 ± 0.69 | 3.62 ± 0.19 | 2.51 ± 0.34 |
| ABS198-4 | 8.31 ± 0.03 | 5.14 ± 0.04 | 3.95 ± 0.46 |
| ABS198-5 | 7.33 ± 0.41 | 3.62 ± 0.04 | 4.75 ± 0.85 |
Data are presented as the average ± SD of three biological replications.
Table 1.
All-trans β-carotene t1/2 with and without HGGT coexpression upon seed storage
| Event with HGGT | All-trans β-carotene, μg/g | α-Tocotrienol, μg/g | α-Tocopherol, μg/g | γ-Tocopherol, μg/g | All-trans β-carotene t1/2, wk | Event without HGGT | All-trans β-carotene, μg/g | All-trans β-carotene t1/2, wk |
| ABS203-1 | 7.31 ± 0.65 | 1.09 ± 0.34 | 2.15 ± 0.54 | 7.96 ± 2.24 | 10.0 ± 0.9 | ABS198-1 | 9.06 ± 1.31 | 3.5 ± 0.4 |
| ABS203-2 | 10.49 ± 0.85 | 1.80 ± 0.72 | 3.07 ± 1.04 | 9.34 ± 1.63 | 9.4 ± 0.8 | ABS198-2 | 5.87 ± 0.37 | 4.4 ± 01.3 |
| ABS203-3 | 8.51 ± 0.92 | 1.27 ± 0.21 | 1.83 ± 0.65 | 8.27 ± 1.04 | 9.4 ± 0.4 | ABS198-3 | 2.51 ± 0.34 | 2.4 ± 0.2 |
| ABS203-4 | 12.30 ± 1.43 | 2.20 ± 0.62 | 3.79 ± 1.14 | 9.55 ± 1.01 | 11.0 ± 1.6 | ABS198-4 | 3.95 ± 0.46 | 4.2 ± 0.7 |
| ABS203-5 | 7.91 ± 0.51 | 1.85 ± 1.44 | 2.12 ± 0.81 | 8.70 ± 2.91 | 10.2 ± 0.6 | ABS198-5 | 4.75 ± 0.85 | 5.5 ± 0.4 |
| ABS203 average | 9.31 | 1.64 | 2.95 | 8.76 | 10.0 | ABS198 average | 5.23 | 3.9 |
| Fold change* | 18.62 | 27.33 | 1.76 | 1.68 | NA | Fold change* | 10.46 | NA |
| WT | 0.50 ± 0.2 | 0.06 ± 0.03 | 1.67 ± 0.25 | 5.21 ± 0.98 | NA | WT | 0.50 ± 0.2 | NA |
Data are presented as the average ± SD of three biological replications.
Compared with WT.
Characterizing the Factors That Affect All-Trans β-Carotene Stability During Seed Storage.
β-Carotene is a highly unsaturated molecule composed of many conjugated double bonds. It is very susceptible to oxidation (32). Many factors affect β-carotene stability, including temperature (thermal degradation), light intensity (photo-oxidative degradation), oxygen level (oxidative degradation), and enzymes (enzymatic oxidative degradation) (33–35).
To study all-trans β-carotene stability and to dissect the causes of degradation during ambient seed storage, we designed and conducted a series of experiments using the multiple-copy ABS168-A event. As shown in Table 2, soaking seeds in water for 30 min at room temperature (21 °C) had a minor effect (4.1% degradation) on the stability of all-trans β-carotene. In contrast, boiling seed for 30 min caused 22.9% degradation, consistent with previous reports (15, 36).
Table 2.
All-trans β-carotene stability of event ABS168-A under different treatments
| Treatment | All-trans β-carotene level after treatment | All-trans β-carotene of the control ± SD after treatments, % | Impact on all-trans β-carotene degradation |
| Seeds stored at −80 °C as control | 9.67 ± 1.04 | 100 | |
| Seeds soaked in water at room temperature in the dark for 30 min and then lyophilized | 9.27 ± 0.69 | 95.9 ± 17.7 | 4.1% degradation with soaking |
| Seeds boiled for 30 min and then lyophilized | 7.46 ± 1.53 | 77.1 ± 24.3 | 22.9% degradation with boiling |
| Seeds stored at room temperature in the dark for 4 wk | 4.65 ± 0.45 | 48.1 ± 9.9 | 51.9% degradation with storage at room temperature |
| Seeds stored at room temperature under constant light (μmol m−2s−1) for 4 wk | 3.61 ± 0.96 | 37.3 ± 14.0 | 10.8% photo-degradation |
| Seeds boiled for 30 min, lyophilized, and then stored at room temperature in dark for 4 wk | 3.23 ± 0.92 | 33.4 ± 13.2 | No detectable enzymatic degradation |
| Seeds sealed in a container purged with pure oxygen at room temperature in the dark for 4 wk | 2.86 ± 0.16 | 29.6 ± 4.9 | Degradation increased from 51.9 to 70.4% |
Data are presented as the average ± SD of three biological replications.
To assay the effect of light on all-trans β-carotene degradation in sorghum grain, ABS168-A grain was stored at room temperature either in the dark or under constant light (at an intensity of μmol m−2s−1) for 4 wk, and degradation data were compared. As shown in Table 2, about 51.9% of all-trans β-carotene was degraded under dark conditions. The degradation increased to 62.7% under light conditions. The slightly increased degradation (10.8%) represented photo-oxidative degradation of all-trans β-carotene over the 4-wk course.
Mature dry sorghum seeds contain about 10% moisture that might maintain limited enzyme activity causing enzymatic degradation of all-trans β-carotene. To test that possibility, ABS168-A seeds were boiled for 30 min to denature all enzymes and then were lyophilized overnight before being stored for 4 wk at room temperature in the dark. Given that boiling degraded all-trans β-carotene by about 22.9%, the actual all-trans β-carotene level before storage was 77.1% of the initial level. Considering that 33.4% of total all-trans β-carotene was retained after storage, the actual degradation caused by storage alone after protein denaturation was 56.7% [(77.1–33.4%)/77.1%] (Table 2), not significantly lower than the 51.9% degradation of all-trans β-carotene caused by storage without boiling treatment. Based on that observation, we concluded that the potential enzymatic degradation in the dry seeds could be ignored.
To determine if oxygen-induced oxidation is the main factor causing degradation, ABS168-A seeds were sealed in a container that was purged with pure oxygen (∼100% O2) to remove the air from the container and were stored for 4 wk at room temperature in the dark. The degradation of all-trans β-carotene increased to 70.4% in this oxygen-enriched environment (Table 2) but was significantly reduced in an oxygen-deprived environment (Fig. 3A).
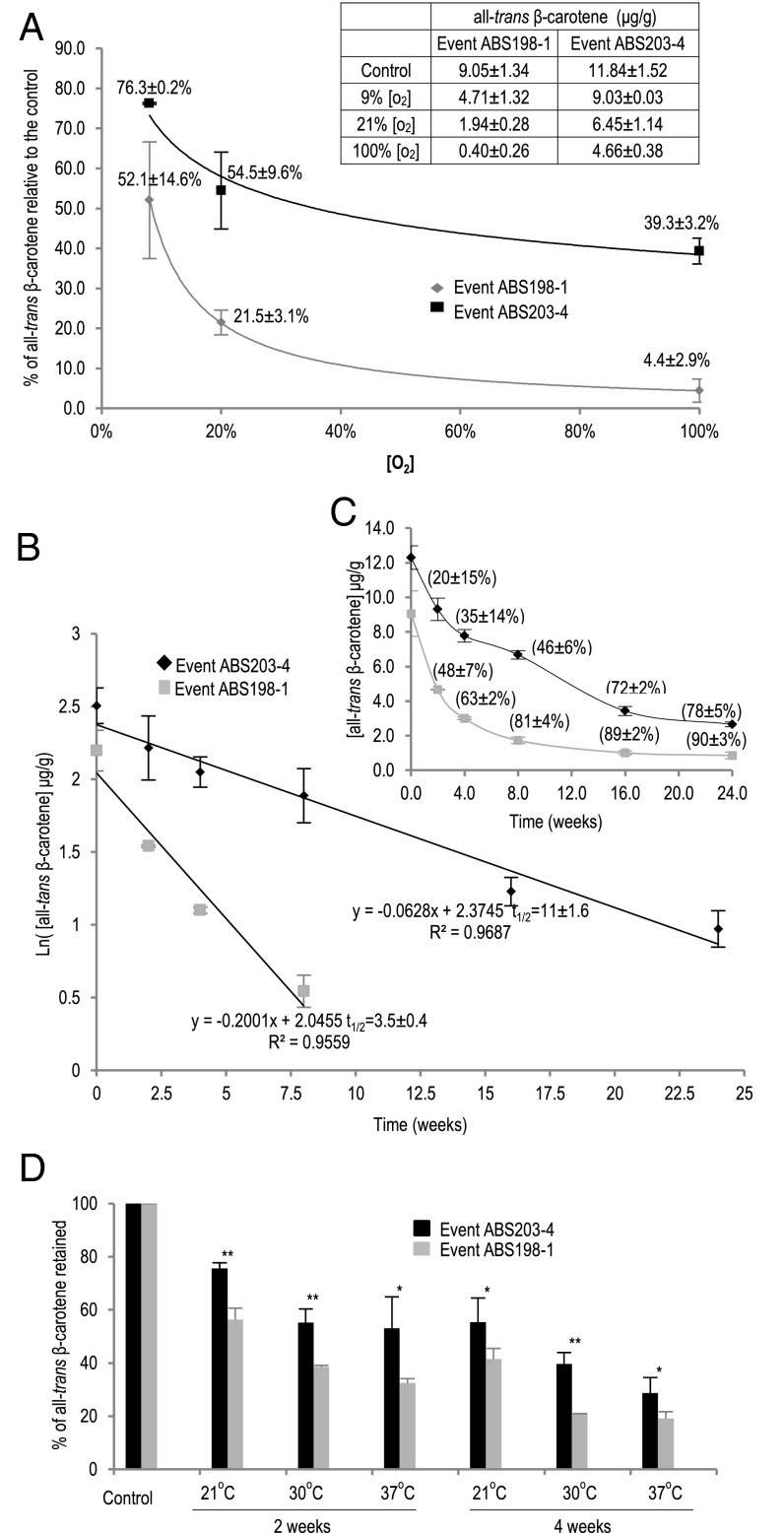
Fig. 3.
The effect of HGGT coexpression on increasing all-trans β-carotene stability during storage. (A) The impact of oxygen concentration on all-trans β-carotene stability. (Inset) The all-trans β-carotene level for each treatment is listed in the table. The control samples were stored at −80 °C. (B) Kinetics of all-trans β-carotene degradation upon ambient storage (see SI Materials and Methods for details). The all-trans β-carotene levels of event ABS198-1 at 16- and 24-wk storage times were too low to be included in the kinetics study. (C) Time course of all-trans β-carotene degradation during ambient seed storage. The percentile numbers represent the percentage of all-trans β-carotene degradation relative to the control seeds stored at −80 °C. (D) The effect of HGGT coexpression on all-trans β-carotene stability at different temperatures. *P < 0.03; **P < 0.002, t test. In all figures, error bars indicate ± SD from three replications.
These results suggested that oxygen-induced oxidative degradation was the main factor contributing to all-trans β-carotene degradation during ambient seed storage.
Improving All-Trans β-Carotene Stability by Ectopic Expression of HGGT.
Vitamin E is a strong antioxidant (37) and is widely used along with β-carotene in the food industry as an additive to increase the shelf-life of β-carotene in foods (38). The biochemical effects of barley HGGT on tocotrienol and tocopherol biosynthesis have been well characterized in planta (26, 27). To prevent the oxidative degradation of all-trans β-carotene in sorghum grain and to increase its stability during seed storage, we introduced the HGGT gene from barley driven with an endosperm-specific promoter along with the carotenoid synthesis genes described for vector–ABS198 and thereby created vector–ABS203 (Fig. 1C). All the top five transgenic ABS203 quality events displayed an orange color (Fig. 2 A and C) in the mature seed endosperms and enhanced all-trans β-carotene accumulation in the range of 7.3–12.3 µg/g DW. ABS203-4 was identified as the best-performing event (Table 1). Other carotenoids, such as lutein, zeathanthin, α-carotene, 13-cis β-carotene, and 9-cis β-carotene, also were increased significantly (Fig. S1 and Tables S1 and S2), with all-trans β-carotene showing the greatest increase both in fold change (24.6-fold) and absolute accumulation (12.3 µg/g DW) (Fig. S1 and Table S1). In addition, both tocotrienols and tocopherols were significantly elevated in these ABS203 events compared with WT (Table 1). The three major vitamin E tocochromanols, α-tocotrienol, α-tocopherol, and γ-tocopherol, were increased 27.33-, 1.76-, and 1.68-fold, respectively. Although α-tocotrienol (1.64 µg/g DW) had the greatest fold-change increase, γ-tocopherol (8.76 µg/g DW) was the most abundant form of vitamin E accumulated in these ABS203 events.
To investigate the antioxidant effect of vitamin E on all-trans β-carotene stability, we compared the stability of all-trans β-carotene in the mature seeds of events ABS198-1 and ABS203-4 treated with different concentrations of oxygen for 4 wk at room temperature in the dark. As demonstrated in Fig. 3A, all-trans β-carotene degradation was increased with the increase of oxygen levels in both ABS198-1 and ABS203-4 events, as was consistent with the observation for event ABS168-A (Table 2). However, the degradation of all-trans β-carotene was much less in event ABS203-4 (with HGGT) than in event ABS198-1 (without HGGT), especially under higher oxygen concentrations. These results further support the hypothesis that oxygen-induced oxidation was the main source of all-trans β-carotene degradation. The antioxidant effect of vitamin E is important in enhancing the stability of all-trans β-carotene under ambient storage conditions.
We studied the kinetics of all-trans β-carotene degradation in sorghum grain during storage at room temperature by determining the relationship between storage time and all-trans β-carotene content at different storage intervals (see Materials and Methods for details). Using the ABS198-1 and ABS203-4 events as examples, the curves of all-trans β-carotene content vs. time represented the time course of all-trans β-carotene degradation in real time (Fig. 3C). The curves were converted into straight lines after the data were replotted with natural-log (ln) all-trans β-carotene content vs. time, indicating that all-trans β-carotene degradation in sorghum grain followed a first-order kinetic model (Fig. 3B) (39–41). The first-order rate constant (k) of β-carotene degradation was determined by the slope of each line, 0.2001/wk for event ABS198-1 and 0.0628/wk for event ABS203-4 (Fig. 3B). Accordingly, the t1/2 of all-trans β-carotene was calculated as 3.5 ± 0.4 wk for event ABS198-1 and 11 ± 1.6 wk for event ABS203-4. As shown in Table 1, the average t1/2 of all-trans β-carotene in the five ABS203 events (10 wk; range, 9.4–11 wk) was significantly longer than in the five ABS198 events (3.9 wk; range, 2.4–5.5 wk). These results showed that the stability of the all-trans β-carotene improved 2.6-fold when HGGT was coexpressed with β-carotene biosynthetic genes in sorghum grain.
To support further the notion that HGGT coexpression can mitigate all-trans β-carotene degradation under different storage temperatures, we measured the degradation of all-trans β-carotene at 30 °C and 37 °C. As shown in Fig. 3D, the higher temperature increased all-trans β-carotene degradation for both ABS198-1 and ABS203-4 events. At both temperatures, however, the degradation of all-trans β-carotene was significantly lower in event ABS203-4 than in event ABS198-1 (either P < 0.03 or P < 0.002).
Collectively, the evidence described above supports the role of HGGT coexpression in increasing the stabilization of all-trans β-carotene during seed storage.
Obtaining Higher All-Trans β-Carotene Accumulation in ABS203 Events.
The decolorization of yellow endosperm during seed maturation in event ABS168-A indicated carotenoid degradation and suggested that the final carotenoid content in the mature sorghum seed could be the result of carotenoid biosynthesis vs. degradation.
Both vector–ABS198 and vector–ABS203 constructs contained exactly the same carotenoid biosynthesis genes (PSY1, CRTI, and At-DXS) regulated with the same promoters. The only difference between these two vectors was that vector–ABS203 contained HGGT. The five best-performing ABS203 quality events contained significantly higher levels of all-trans β-carotene (9.3 µg/g DW by average) in the mature seeds than did the five best-performing ABS198 quality events (5.4 µg/g DW by average) (Table 1). Considering the potential for all-trans β-carotene degradation during seed maturation as indicated by the color change in event ABS168-A, we hypothesized that coexpressing HGGT could mitigate all-trans β-carotene degradation during seed maturation.
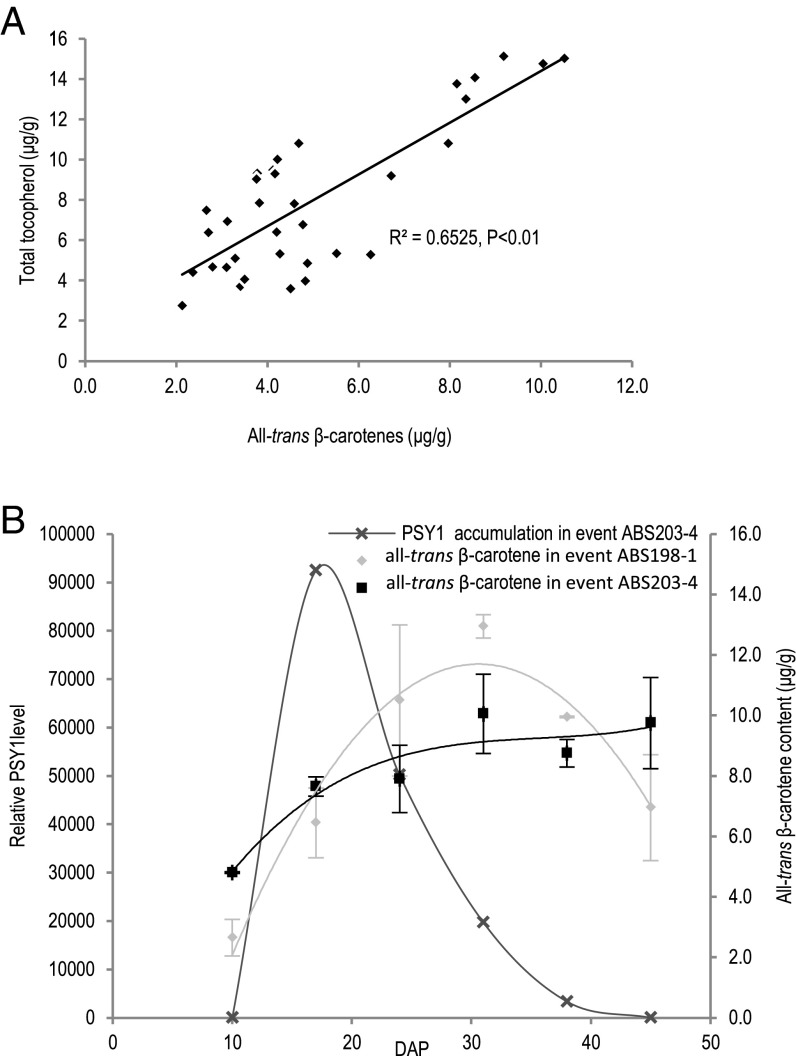
To test this hypothesis, we investigated the interrelationship between carotenoids and vitamin E content in mature seeds collected from 35 independent transgenic ABS203 plants. As shown in Fig. 4A, we observed a positive correlation (R2 = 0.6525, P < 0.01) between all-trans β-carotene and total tocopherol (α- and γ-tocopherol) content in these 35 independent transgenic plants. This observation indicated that the antioxidant function of vitamin E could increase the stability of all-trans β-carotene through seed maturation, potentially leading to a higher accumulation of all-trans β-carotene in the mature seeds.
Fig. 4.
The effect of HGGT coexpression on increasing all-trans β-carotene accumulation during seed development. (A) The positive correlation between the accumulation of all-trans β-carotene and total tocopherols in mature event ABS203 seeds harvested from 35 independent plants. (B) The relationship between the accumulation of all-trans β-carotene and PSY1 protein during seed development in event ABS198-1 and ABS203-4. A similar pattern of PSY1 protein accumulation was observed in two other ABS203 events (Fig. S2). Error bars indicate ± SD from three replications.
Fig. S2.
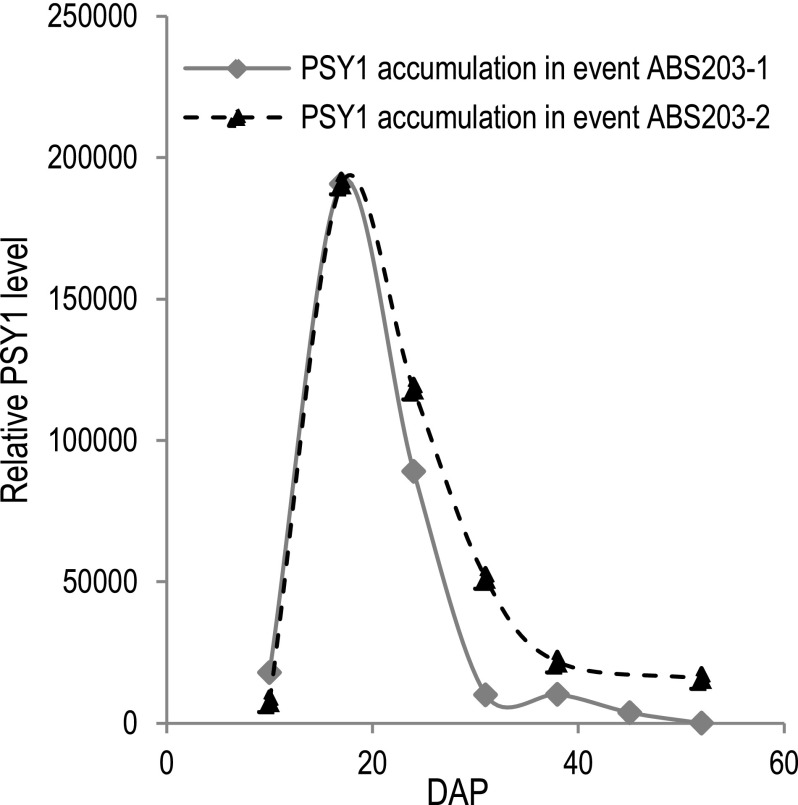
Patterns of PSY1 protein accumulation during seed development in events ABS203-1 and ABS203-2. Samples were collected at six or seven stages of seed development (10, 17, 24, 31, 38, 45, and 52 DAP).
To gain insight into all-trans β-carotene accumulation throughout seed development, we determined the accumulation of PSY1 protein (the enzyme that controls the rate-limiting step of carotenoid biosynthesis) by LC-MS/MS at six stages of seed development (10, 17, 24, 30, 38, and 45 DAP) in event ABS203-4. We found that PSY1 accumulated to the highest levels at the milk-stage (around 17 DAP) and declined sharply to an undetected level at maturity (around 45 DAP) (Fig. 4B), indicating that the capacity for carotenoid biosynthesis was higher at the early stages and was lower at the later stages of seed development. Because PSY1 expression was driven by the same endosperm-specific promoter in ABS198 events and ABS203 events, it was reasonable to speculate that PSY1 accumulation followed a similar pattern in ABS198 and ABS203 events during seed development. To investigate the correlation between PSY1 accumulation and the all-trans β-carotene levels during the seed development, we further measured the accumulation of all-trans β-carotene at these six stages and found the highest level of all-trans β-carotene accumulation around 31 DAP for both event ABS203-4 and ABS198-1 (Fig. 4B). Afterward, the all-trans β-carotene levels declined sharply in event ABS198-1, similar to the previous report (10), but not in event ABS203-4. The sharp decline of all-trans β-carotene accumulation in event ABS198-1 correlated with a reduction in PSY1 accumulation, suggesting that all-trans β-carotene degradation surpassed the rate of biosynthesis at around 31 DAP in event ABS198-1. On the other hand, all-trans β-carotene accumulation remained constant through seed maturity in event ABS203-4, indicating that HGGT coexpression during seed development can mitigate all-trans β-carotene degradation in ABS203 events.
Discussion
Many attempts have been made to alter or to enhance the carotenoid biosynthetic pathways in various plant tissues (4, 12–15, 22), but little attention has been paid to carotenoid stability, especially all-trans β-carotene stability, in biofortified crops. The success of GR2 researchers in manipulating de novo carotenoid biosynthesis resulted in higher levels of β-carotene in rice endosperm (28), but the carotenoids degraded when Golden Rice was stored at ambient temperatures (42). Similarly, ectopic expression of the enzymes involved in carotenoid biosynthesis in sorghum endosperm resulted in increased all-trans β-carotene levels but with a t1/2 of less than 4 wk (Figs. 2A and 4B and Table 1). Because sorghum grain must be stored and consumed over several months before newly harvested grain is available in most sub-Saharan Africa countries, developing a way to make all-trans β-carotene more stable during storage in ambient conditions is important for the full utilization of biofortified sorghum to deliver essential micronutrients. The focus of this research, therefore, was to develop technologies that not only increase all-trans β-carotene accumulation in sorghum grain but also make all-trans β-carotene more stable during seed storage.
The mechanisms of carotenoid metabolism have been well studied (43, 44). Factors identified as influencing carotenoid degradation in foods are oxygen, temperature, light, pH, ionic strength, moisture, and food matrix (33–35). In biofortified sorghum, oxygen-induced degradation is the greatest factor in all-trans β-carotene degradation during ambient seed storage (Fig. 3A and Table 2). Although vacuum sealing β-carotene–enriched grain potentially could slow β-carotene degradation, as demonstrated in Fig. 3A, this technique of storing biofortified sorghum is not practical for smallholder farmers in African countries. In the food industry, vitamin E has been shown to be the most effective antioxidant for preventing β-carotene degradation in dehydrated vegetables and fruits (45). Coexpression of HGGT to increase vitamin E biosynthesis, together with all-trans β-carotene biosynthesis, can mitigate the oxidative degradation of all-trans β-carotene (Fig. 3 B–D). The t1/2 of all-trans β-carotene was extended significantly, from less than 4 wk to 10 wk on average, with coexpression of HGGT (Table 1). We observed the degradation of all three forms of vitamin E (α-tocotrienol, α-tocopherol, and γ-tocopherol) during seed storage, as shown in Table S3. This was correlated with an attenuated degradation of β-carotene during seed storage, suggesting that vitamin E may be protecting β-carotene from oxidative degradation. Although degradation was not completely prevented, we believe that the stability of all-trans β-carotene can be improved further by increasing vitamin E accumulation through the use of stronger endosperm-specific or even whole-seed promoters for ectopic expression of HGGT. In addition to preventing the degradation of all-trans β-carotene during mature seed storage, HGGT coexpression can prevent such degradation during seed development, resulting in higher all-trans β-carotene accumulation in freshly harvested grain (Fig. 4B).
Table S3.
Comparison of the stability of vitamin E and all-trans β-carotene during seed storage
| Event ABS203-4 | α-Tocotrienol | α-Tocopherol | γ-Tocopherol | All-trans β-carotene |
| Initial content, μg/g | 1.12 ± 0.19 | 2.42 ± 0.25 | 10.16 ± 0.4 | 11.84 ± 1.52 |
| Content after 4 wk storage at room temperature, μg/g | 0.53 ± 0.08 | 1.03 ± 0.12 | 8.2 ± 0.3 | 6.45 ± 1.14 |
| Retention at fourth week of storage, % | 43 ± 7.1 | 42.7 ± 12.7 | 80.9 ± 5.4 | 54.5 ± 1.7 |
Data are presented as the average ± SD of three biological replications.
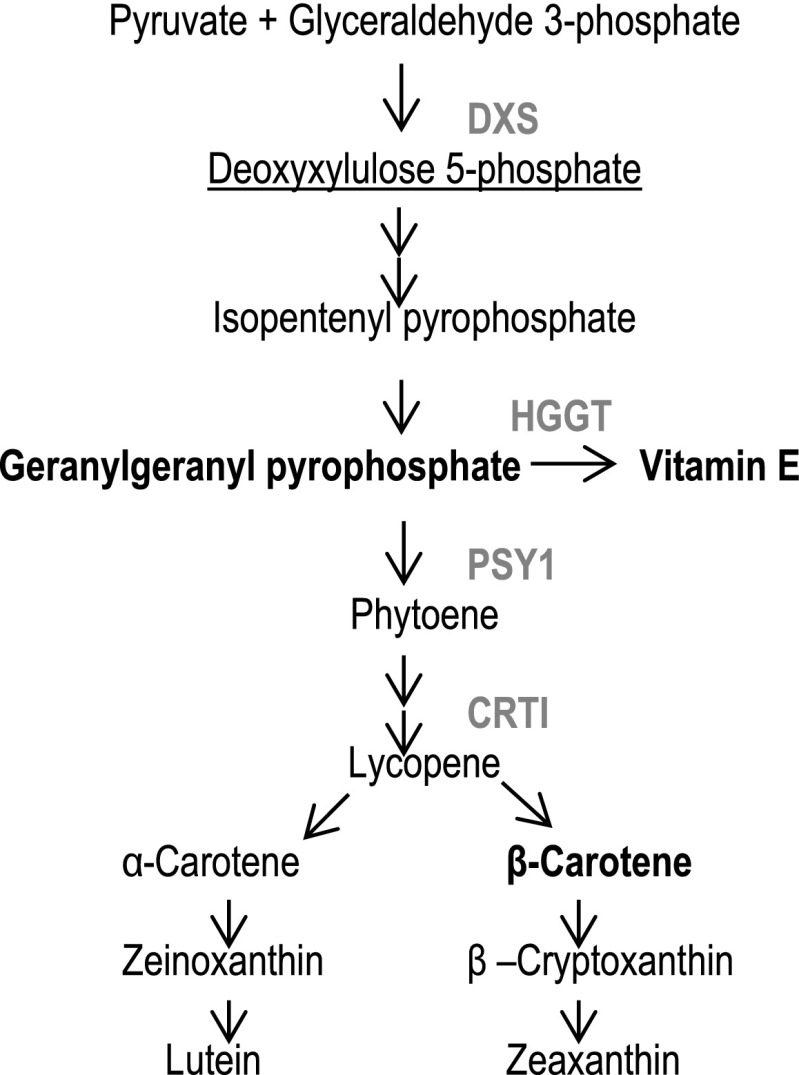
Understanding how β-carotene biosynthesis (Fig. S3) and accumulation are regulated during seed development has helped in the design of new approaches to achieve higher β-carotene accumulation in mature sorghum grain. The correlation between all-trans β-carotene and PSY1 enzyme accumulation during seed development in event ABS198 (Fig. 4B) further supports the notion that PSY1 controls the rate-limiting step of carotenoid biosynthesis and provides insight into ways of further increasing β-carotene biosynthesis. If PSY1 gene expression and protein accumulation can be kept consistently at relatively higher levels throughout the later stages of seed development (e.g., by the use of promoters that achieve higher PSY1 expression at all seed developmental stages, monocot codon optimization, or the selection of more stable and efficient versions of PSY), biofortified sorghum grain will accumulate more β-carotene in mature seeds. These methods combined with the recent discovery that carotenoid biosynthesis is controlled via posttranscriptional regulation of PSY by ORANGE could further improve β-carotene biosynthesis in sorghum grain (46).
Fig. S3.
Highlights of the enzymes used for sorghum biofortification in the carotenoid biosynthesis pathway.
A further role of α-tocopherol in promoting the central cleavage of the β-carotene molecule to form vitamin A rather than β-apo carotenoids was demonstrated using rat intestinal postmitochondrial fractions incubated with β-carotene (47). The multiple functions of vitamin E not only increase β-carotene stability by preventing oxidation during storage but also potentially increase the efficiency of vitamin A conversion by promoting central cleavage of the β-carotene molecule during consumption. Combining vitamin E biosynthesis with enhanced all-trans β-carotene biosynthesis will enable the development of biofortified sorghum that can eliminate vitamin A deficiency in people who consume sorghum as their staple diet. Based on the β-carotene bioconversion rate (4.3 µg β-carotene to 1 µg retinol) determined for event ABS203-4 using Mongolian gerbils as an animal model (48) and based on the stability of β-carotene determined in this research, we estimated that freshly harvested β-carotene–biofortified sorghum from this event could provide 90% of the estimated average requirement (EAR) of vitamin A for children under age 3 y and still would provide 20% of EAR after 6 mo of seed storage. On the other hand, event ABS198-1 would provide only 8% of EAR after 6 mo of seed storage.
Materials and Methods
Vector construction, transgenic events, treatments of sorghum seeds, kinetic studies of all-trans β-carotene degradation, HPLC and LC-MS/MS assays, and statistical analysis are described in SI Materials and Methods.
SI Materials and Methods
Constructs for Transformation.
The combination of maize PSY1 (28), Pantoea ananatis CRTI (49), Arabidopsis DXS (30), barley HGGT (26, 27), and Escherichia coli phosphomannose isomerase (PMI) (50) genes were synthesized and cloned into JT superbinary vector pSB1 (51, 52) to construct vector–ABS168, vector–ABS198, and vector–ABS203 as illustrated in Fig. 1.
For vector–ABS168, the Sorghum bicolor α-kafirin (SB-AKAF) promoter from the SB-BKAF gene was operationally linked to PSY1. Similarly, a nucleic acid molecule encoding a fusion of the small subunit gene chloroplast transit peptide (SSU TP) coding sequence of pea was linked with the coding sequence of the CRTI after the SB-BKAF promoter. Both cassettes were stacked using Gateway recombinational cloning into JT superbinary vector pSB1 comprising a plant-selectable marker gene, PMI, which was driven by the maize UBI promoter and intron 1.
Vector–ABS198 contained all the gene cassettes in vector–ABS168 plus an additional gene cassette comprising the GZ-W64A promoter of maize γ-zein gene operably linked to the Arabidopsis thaliana At-DXS coding sequence. Vector–ABS203 was constructed using the gene cassettes used in vector–ABS198 plus a cassette comprising the SB-AKAF A1 promoter operably linked to the barley HGGT coding sequence. The functions of these genes in the carotenoid biosynthesis pathway are illustrated in Fig. S3.
Sorghum Transformation, Quantitative PCR Analysis, and Transgenic Materials Used.
Greenhouse-grown sorghum genotype TX430 was used in this study. Immature embryo explants isolated from TX430 plants were transformed with Agrobacterium LAB4404 carrying the vectors described above to generate transgenic sorghum plants. The Agrobacterium transformation protocol was described in detail previously (25). The integrated copy number of the T-DNA and the vector backbone in these transgenic plants were determined by a series of quantitative PCR (qPCR) analyses (25). The initial transgenic plants carrying a single copy of the intact T-DNA integration without vector backbone were defined as “quality events.” For each vector, 20 quality events were produced through sorghum transformation to ensure that the materials used in this study represented a reasonable sample size. These transgenic events were self-pollinated to generate a mix of transgenic seeds and nontransgenic seeds in a ratio of 3:1 verified by qPCR of the PMI. This 3:1 segregation ratio further confirmed a single insertion site in the sorghum genome. The homozygosity of the transgenes in the following generation also was determined by qPCR. The homozygous events were self-pollinated to generate 100% homozygous transgenic seeds from each individual plant. If an initial transgenic plant carried multiple insertions (more than one copy based on qPCR analysis) of the T-DNA, and all the copies were intact, this plant was defined as a “multiple-copy event.” A transgenic plant originally derived from a single embryo and its progeny were defined as an “independent transgenic event.” In general, an independent transgenic event had a unique T-DNA integration site in the sorghum genome and a unique transgene-expression profile.
In this study, homozygous transgenic seeds from five independent ABS198 and ABS203 transgenic quality events were used to represent their biological variations in most of the experiments. The event ABS168-A used in this study carried multiple copies of the T-DNA. Also, for all events derived from these three vectors, the best-performing events were chosen to represent the maximum capacity of the gene functions in sorghum. The best-performing events were selected based on the levels of all-trans β-carotene in their seeds and normal plant phenotypes.
Sorghum panicles were collected from sorghum plants at 45 DAP and were air dried at 24 °C for 2 wk before threshing. The threshed seeds (about 10% moisture) were stored in a freezer at −80 °C before any experiments were conducted.
Treatments of Sorghum Seeds and Statistical Analysis.
Different concentrations of oxygen (100, 21, and 9%) were achieved by purging a sealed flask containing the seeds with pure oxygen (100% O2), exposing the seeds to ambient oxygen (21%), or drawing down the air pressure in the flask to about 43% atmospheric pressure (9% O2). Different temperature treatments were conducted by leaving seeds either at room temperature (21 °C) or in incubators set at the required temperatures (30 or 37 °C).
In all experiments, three seed samples randomly picked from the seeds derived from an independent transgenic event were treated in the same conditions at the same time and then were analyzed along with samples from other treatments in the same experiment. The samples were renumbered for analysis to ensure blind testing. The three samples from one event represented technical replications to ensure that the treatments and analyses were reproducible.
The one-sided t test was used for all statistical analysis, and the SD was calculated based on the three replicated samples in each event. The mean from these samples with its SD were used to represent a data point.
The Kinetics of All-Trans β-Carotene Degradation in Sorghum Grains.
The first-order kinetics model of β-carotene degradation was reported previously (39–41). An empirical first-order kinetics formula was used for the degradation kinetics of β-carotene: lnC = lnC0 − (k)(t), where C0 (in micrograms per gram) is the initial quantity of β-carotene in sorghum grain, C (in micrograms per gram) is the quantity of β-carotene at time t (in weeks) after storage, and k is the reaction rate constant (per week). The t1/2 times of β-carotene were calculated according to the equation t1/2 = ln2/k. To characterize the kinetics of all-trans β-carotene degradation and to determine the t1/2 of all-trans β-carotene degradation in sorghum grain during storage, sorghum seeds derived from five independent ABS198 and ABS203 events, as indicated in Table 1, were left at room temperature in the dark for different time intervals (0, 2, 4, 8, 16, and 24 wk) and then were stored at −80 °C before HPLC analysis of all-trans β-carotene content. The kinetics of all-trans β-carotene degradation was determined by plotting the natural-log (ln) all-trans β-carotene content vs. time. The linear correlation between the data obtained was judged by the coefficient of determination (R2).
HPLC.
HPLC was used for analysis of carotenoids. All extraction procedures were completed under low light to minimize the potential for photo-oxidative reactions, and carotenoids were analyzed by HPLC with UV detection. Sorghum seeds were ground with a Geno grinder, and the weight of the ground material was recorded. The ground material was extracted with 1 mL cold water and then with 12 mL cold acetone. The HPLC analysis used to detect carotenoids in the test cross progenies was a modification of methods described previously (28) using a Waters YMC Carotenoid 3-μm (4.6 × 150 mm) column or equivalent and a Waters 2487 UV Detector or equivalent. Samples were loaded into an amber glass autosampler vial, and carotenoids were detected at 450 nm at a flow rate of 2 mL/min of 75% buffered methanol and 25% ethyl acetate, with each run taking 25 min. Quantification of compounds was accomplished by standard regression with external standards. Apocarotenal was used as internal control. The carotenoid contents in sorghum grains were reported as micrograms per gram DW for data analysis. Detailed carotenoid compositions are shown in Fig. S1 and Table S1.
HPLC was also used for tocochromanols analysis. The tocotrienols and tocopherols were determined as described previously (53). Sorghum seeds were ground with a Geno grinder. After the weight of the ground material was recorded, the ground material was extracted with 2 mL of heptane under reduced lighting. Tocochromanols were separated by using a Waters HPLC Alliance 2695 with a 3-μm NH2 100 A, 150 mm × 3.0 mm column or equivalent and were detected by fluorescence (Waters 2475 or equivalent) with excitation λ = 292 nm and emission λ = 335 nm. An external calibration curve of 0.05, 0.1, 0.2, 0.5, 1.0, 2.5, and 5 ppm of each tocotrienol and tocopherol was used for quantification. The tocotrienol and tocopherol contents in sorghum grains were expressed as micrograms per gram DW.
Determination of Relative PSY1 Protein Accumulation During Seed Development.
The relative levels of PSY1 protein accumulation were estimated by using LC-MS/MS according to ref. 54. After being lyophilized and ground, 10 mg of samples were extracted with 500 µL ACT buffer [10 mM ammonium bicarbonate with 0.05% CHAPS and 0.01% (vol/vol) Triton X-100]. After centrifuging, 100 µL of extracts were reduced with 6 µL of 0.25 M DTT at 50 °C for 30 min and then were alkylated with 6 µL of 0.3 M iodoacetamide at room temperature in the dark for 30 min. Four micrograms of trypsin (10 µL) were added to each sample, and digestion was carried out in a CEM Discover Proteomics System at 45 °C, 50 W for 30 min before 10 μL of 10% (vol/vol) formic acid was added. PSY1 protein was estimated by monitoring its signature tryptic peptide FPIDIQPFR with a multiple reaction monitoring (MRM) transition of 566.8/660.3, using Waters UPLC coupled with AB SCIEX Q-TRAP 5500. The autosampler temperature was maintained at 8 °C during analysis. Ten-microliter volumes were injected onto an Aquasil 100 × 2.1 mm 3-µm C18 column (Thermo Fisher) maintained at 60 °C. Mobile phases consisted of 0.1% formic acid (mobile phase A) and 0.1% formic acid in acetonitrile (mobile phase B, MPB), and LC was performed at a flow rate of 0.25 mL/min with a linear gradient of 2–35% MPB in 32 min.
When performing all the analyses described above, investigators were blinded to sample identities, and samples were tracked with sample identifications that did not link to the sample information.
Acknowledgments
We thank Edgar B. Cahoon (University of Nebraska) for vitamin E validation; Heather Christenson, Tracy Asmus, David Draize, and Weiwei Zhu (DuPont Pioneer) for sorghum transformation; Brian Lenderts and Nancy Leysens (DuPont Pioneer) for quantitative PCR analysis; Mark Perkins (DuPont Pioneer) for HPLC analysis; Shifu Zhen (DuPont Pioneer) for greenhouse management; and the Donald Danforth Plant Science Center for certain funding administration. This research was supported by Bill and Melinda Gates Foundation Grand Challenges in Global Health Grant ID-37877 and by The Howard G. Buffett Foundation funding of the Africa Biofortified Sorghum Project. DuPont Pioneer provided funding and in-kind donations.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1605689113/-/DCSupplemental.
References
- 1.Beaton GH, et al. 1993. Effectiveness of Vitamin A Supplementation in the Control of Young Child Morbidity and Mortality in Developing Countries. Nutrition Policy Discussion Paper No. 13. (United Nations Administrative Committee on Coordination, Subcommittee on Nutrition, Geneva)
- 2.Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: A global perspective. Bull World Health Organ. 2001;79(3):214–221. [PMC free article] [PubMed] [Google Scholar]
- 3.Food and Nutrition Board of the Institute of Medicine 2001. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. (National Academy Press, Washington, DC)
- 4.Van Loo-Bouwman CA, Naber TH, Schaafsma G. A review of vitamin A equivalency of β-carotene in various food matrices for human consumption. Br J Nutr. 2014;111(12):2153–2166. doi: 10.1017/S0007114514000166. [DOI] [PubMed] [Google Scholar]
- 5.van de Pavert SA, et al. Maternal retinoids control type 3 innate lymphoid cells and set the offspring immunity. Nature. 2014;508(7494):123–127. doi: 10.1038/nature13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eberl G. Immunology: A is for immunity. Nature. 2014;508(7494):47–48. doi: 10.1038/nature13216. [DOI] [PubMed] [Google Scholar]
- 7.Report G. 2009 Investing in the future - A united call to action on vitamin and mineral deficiencies. Available at www.unitedcalltoaction.org/index.asp. Accessed August 30, 2016.
- 8.Duodu KG, Taylor JRN, Belton PS, Hamaker BR. Factors affecting sorghum protein digestibility. J Cereal Sci. 2003;38(2):117–131. [Google Scholar]
- 9.Shewry PR. Improving the protein content and composition of cereal grain. J Cereal Sci. 2007;46(3):239–250. [Google Scholar]
- 10.Kean EG, Ejeta G, Hamaker BR, Ferruzzi MG. Characterization of carotenoid pigments in mature and developing kernels of selected yellow-endosperm sorghum varieties. J Agric Food Chem. 2007;55(7):2619–2626. doi: 10.1021/jf062939v. [DOI] [PubMed] [Google Scholar]
- 11.Hefferon KL. Nutritionally enhanced food crops; progress and perspectives. Int J Mol Sci. 2015;16(2):3895–3914. doi: 10.3390/ijms16023895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brinch-Pedersen H, Borg S, Tauris B, Holm P. Molecular genetic approaches to increasing mineral availability and vitamin content of cereals. J Cereal Sci. 2007;46(3):308–326. [Google Scholar]
- 13.Hotz C, McClafferty B. From harvest to health: Challenges for developing biofortified staple foods and determining their impact on nutrient status. Food Nutr Bull. 2007;28(Suppl):271–279. doi: 10.1177/15648265070282S206. [DOI] [PubMed] [Google Scholar]
- 14.Giuliano G, Tavazza R, Diretto G, Beyer P, Taylor MA. Metabolic engineering of carotenoid biosynthesis in plants. Trends Biotechnol. 2008;26(3):139–145. doi: 10.1016/j.tibtech.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 15.De Moura FF, Miloff A, Boy E. Retention of provitamin a carotenoids in staple crops targeted for biofortification in Africa: Cassava, maize and sweet potato. Crit Rev Food Sci Nutr. 2015;55(9):1246–1269. doi: 10.1080/10408398.2012.724477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krishnamurthy DSMR. Alleviation of malnutrition by biofortification of crops. International Journal of Humanities and Social Science Invention. 2013;2(5):1–8. [Google Scholar]
- 17.Zhu C, et al. Biofortification of plants with altered antioxidant content and composition: Genetic engineering strategies. Plant Biotechnol J. 2013;11(2):129–141. doi: 10.1111/j.1467-7652.2012.00740.x. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization 2014 Biofortification of staple crops, e-library of evidence for nutrition actions (eLENA). Available at www.who.int/elena/titles/biofortification/en/. Accessed August 30, 2016.
- 19.Murgia I, De Gara L, Grusak MA. Biofortification: How can we exploit plant science and biotechnology to reduce micronutrient deficiencies? Front Plant Sci. 2013;4(429):1–3. doi: 10.3389/fpls.2013.00429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blancquaert D, De Steur H, Gellynck X, Van Der Straeten D. Present and future of folate biofortification of crop plants. J Exp Bot. 2014;65(4):895–906. doi: 10.1093/jxb/ert483. [DOI] [PubMed] [Google Scholar]
- 21.Kabir AH, Swaraz AM, Stangoulis J. Zinc-deficiency resistance and biofortification in plants. J Plant Nutr Soil Sci. 2014;177(3):311–319. [Google Scholar]
- 22.Bhullar NK, Gruissem W. Nutritional enhancement of rice for human health: The contribution of biotechnology. Biotechnol Adv. 2013;31(1):50–57. doi: 10.1016/j.biotechadv.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 23.Zhao ZY, et al. Nutritionally improved transgenic sorghum. In: Vasil IK, editor. Plant Biotechnology 2002 and Beyond. Kluwer; Dordrecht, The Netherlands: 2003. pp. 413–416. [Google Scholar]
- 24.da Silva LS, et al. Effect of suppressing the synthesis of different kafirin sub-classes on grain endosperm texture, protein body structure, and protein nutritional quality in improved sorghum lines. J Cereal Sci. 2011;54(1):160–167. [Google Scholar]
- 25.Wu E, et al. Optimized Agrobacterium-mediated sorghum transformation protocol and molecular data of transgenic sorghum plants. In Vitro Cell Dev Biol Plant. 2014;50(1):9–18. doi: 10.1007/s11627-013-9583-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cahoon EB, et al. Metabolic redesign of vitamin E biosynthesis in plants for tocotrienol production and increased antioxidant content. Nat Biotechnol. 2003;21(9):1082–1087. doi: 10.1038/nbt853. [DOI] [PubMed] [Google Scholar]
- 27.Cahoon EB, Coughlan SJ, Cahoon RE, Butler KH. 2006. Compositions and methods for altering tocotrienal content. US Patent 7,154,029 B2.
- 28.Paine JA, et al. Improving the nutritional value of Golden Rice through increased pro-vitamin A content. Nat Biotechnol. 2005;23(4):482–487. doi: 10.1038/nbt1082. [DOI] [PubMed] [Google Scholar]
- 29.Lipkie TE, et al. Bioaccessibility of carotenoids from transgenic provitamin A biofortified sorghum. J Agric Food Chem. 2013;61(24):5764–5771. doi: 10.1021/jf305361s. [DOI] [PubMed] [Google Scholar]
- 30.Estévez JM, Cantero A, Reindl A, Reichler S, León P. 1-Deoxy-D-xylulose-5-phosphate synthase, a limiting enzyme for plastidic isoprenoid biosynthesis in plants. J Biol Chem. 2001;276(25):22901–22909. doi: 10.1074/jbc.M100854200. [DOI] [PubMed] [Google Scholar]
- 31.Lois LM, Rodríguez-Concepción M, Gallego F, Campos N, Boronat A. Carotenoid biosynthesis during tomato fruit development: Regulatory role of 1-deoxy-D-xylulose 5-phosphate synthase. Plant J. 2000;22(6):503–513. doi: 10.1046/j.1365-313x.2000.00764.x. [DOI] [PubMed] [Google Scholar]
- 32.Delgado-Vargas F, Jiménez AR, Paredes-López O. Natural pigments: Carotenoids, anthocyanins, and betalains--characteristics, biosynthesis, processing, and stability. Crit Rev Food Sci Nutr. 2000;40(3):173–289. doi: 10.1080/10408690091189257. [DOI] [PubMed] [Google Scholar]
- 33.Meléndez-Martínez AJ, Vicario IM, Heredia FJ. [Stability of carotenoid pigments in foods] Arch Latinoam Nutr. 2004;54(2):209–215. [PubMed] [Google Scholar]
- 34.Boon CS, McClements DJ, Weiss J, Decker EA. Factors influencing the chemical stability of carotenoids in foods. Crit Rev Food Sci Nutr. 2010;50(6):515–532. doi: 10.1080/10408390802565889. [DOI] [PubMed] [Google Scholar]
- 35.Qian C, Decker EA, Xiao H, McClements DJ. Physical and chemical stability of β-carotene-enriched nanoemulsions: Influence of pH, ionic strength, temperature, and emulsifier type. Food Chem. 2012;132(3):1221–1229. doi: 10.1016/j.foodchem.2011.11.091. [DOI] [PubMed] [Google Scholar]
- 36.Li S, Tayie FAK, Young MF, Rocheford T, White WS. Retention of provitamin A carotenoids in high beta-carotene maize (Zea mays) during traditional African household processing. J Agric Food Chem. 2007;55(26):10744–10750. doi: 10.1021/jf071815v. [DOI] [PubMed] [Google Scholar]
- 37.Brigelius-Flohé R, Traber MG. Vitamin E: Function and metabolism. FASEB J. 1999;13(10):1145–1155. [PubMed] [Google Scholar]
- 38.Choe E, Min DB. Mechanisms of antioxidants in the oxidation of foods. Compr Rev Food Sci Food Saf. 2009;8(4):345–358. [Google Scholar]
- 39.Henry LK, Catignani GL, Schwartz SJ. Oxidative degradation kinetics of lycopene, lutein, and 9-cis and all-trans β-carotene. J Am Oil Chem Soc. 1998;75(7):823–829. [Google Scholar]
- 40.Demiray E, Tulek Y, Yilmaz Y. Degradation kinetics of lycopene, β-carotene and ascorbic acid in tomatoes during hot air drying. LWT-Food Sci Technol (Campinas) 2013;50(1):172–176. [Google Scholar]
- 41.Goula AM, Adamopoulos KG. Kinetic models of β-carotene degradation during air drying of carrots. Dry Technol. 2010;28(6):752–761. [Google Scholar]
- 42.Pham TN, Dong TL, Tran VH, Tran TCH. Effect of storage conditions on total carotenoid content in golden rice grains. Omonrice. 2006;14:18–27. [Google Scholar]
- 43.Nisar N, Li L, Lu S, Khin NC, Pogson BJ. Carotenoid metabolism in plants. Mol Plant. 2015;8(1):68–82. doi: 10.1016/j.molp.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 44.Moise AR, Al-Babili S, Wurtzel ET. Mechanistic aspects of carotenoid biosynthesis. Chem Rev. 2014;114(1):164–193. doi: 10.1021/cr400106y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Y, Hou Z, Yang J, Gao Y. Effects of antioxidants on the stability of β-Carotene in O/W emulsions stabilized by gum arabic. J Food Sci Technol. 2015;52(6):3300–3311. doi: 10.1007/s13197-014-1380-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou X, et al. Arabidopsis OR proteins are the major posttranscriptional regulators of phytoene synthase in controlling carotenoid biosynthesis. Proc Natl Acad Sci USA. 2015;112(11):3558–3563. doi: 10.1073/pnas.1420831112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yeum KJ, dos Anjos Ferreira AL, Smith D, Krinsky NI, Russell RM. 2000. The effect of alpha-tocopherol on the oxidative cleavage of beta-carotene. 29(2):105–114. [DOI] [PubMed]
- 48.You H, et al. 2015. Quantifying the bioefficacy of β-carotene-biofortified sorghum using a Mongolian gerbil model. FASEB J 29(1):Supplement 605.3.
- 49.Ye X, et al. Engineering the provitamin A (beta-carotene) biosynthetic pathway into (carotenoid-free) rice endosperm. Science. 2000;287(5451):303–305. doi: 10.1126/science.287.5451.303. [DOI] [PubMed] [Google Scholar]
- 50.Miles JS, Guest JR. Nucleotide sequence and transcriptional start point of the phosphomannose isomerase gene (manA) of Escherichia coli. Gene. 1984;32(1-2):41–48. doi: 10.1016/0378-1119(84)90030-1. [DOI] [PubMed] [Google Scholar]
- 51.Komari T. Transformation of cultured cells of Chenopodium quinoa by binary vectors that carry a fragment of DNA from the virulence region of pTiBo542. Plant Cell Rep. 1990;9(6):303–306. doi: 10.1007/BF00232856. [DOI] [PubMed] [Google Scholar]
- 52.Komari T, Hiei Y, Saito Y, Murai N, Kumashiro T. Vectors carrying two separate T-DNAs for co-transformation of higher plants mediated by Agrobacterium tumefaciens and segregation of transformants free from selection markers. Plant J. 1996;10(1):165–174. doi: 10.1046/j.1365-313x.1996.10010165.x. [DOI] [PubMed] [Google Scholar]
- 53.Dolde D, Wang T. Oxidation of crude corn oil with and without elevated tocotrienols. J Am Oil Chem Soc. 2011;88(9):1367–1372. [Google Scholar]
- 54.Hu XT, Owens MA. Multiplexed protein quantification in maize leaves by liquid chromatography coupled with tandem mass spectrometry: An alternative tool to immunoassays for target protein analysis in genetically engineered crops. J Agric Food Chem. 2011;59(8):3551–3558. doi: 10.1021/jf104516r. [DOI] [PubMed] [Google Scholar]