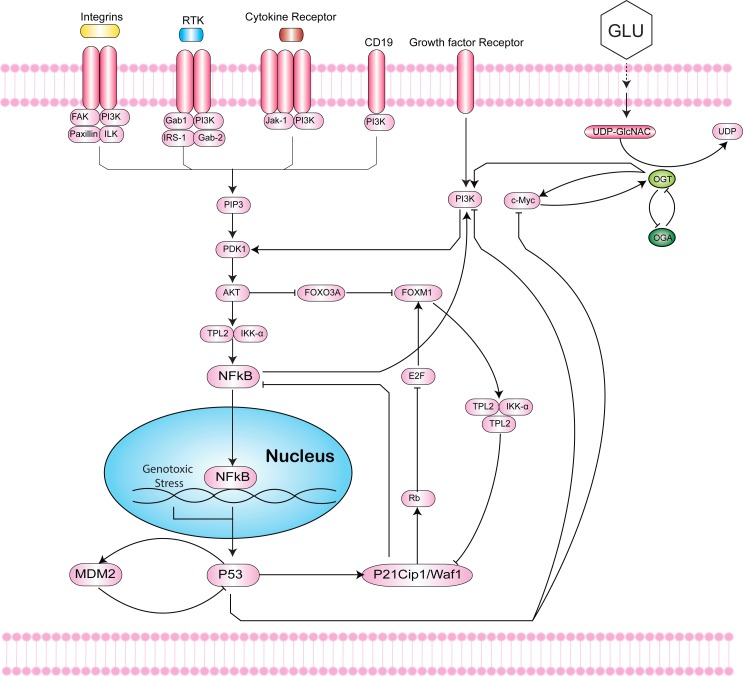
Figure 1. Intersection of the Hexosamine Biosynthetic Pathway (HBP), Phosphoinositide 3-kinase (PI3K)-mTOR-MYC signaling axis, and p53-MDM2 circuit.
The HBP (right) generates Uridine diphosphate N-acetylglucosamine (UDP-GlcNAc) as the end product that is used by the O-GlcNAc transferase (OGT) to covalently attach O-GlcNAc to hydroxyl groups of serine/threonine residues of proteins. This dynamic process is antagonized by O-GlcNAcase (OGA). In cancer, increased HBP flux leads to hyper O-GlcNAcylation. Hyper O-GlcNAcylation of c-Myc activates the phosphoinositide 3-kinase (PI3K)-mTOR-MYC signaling axis (middle). The PI3K pathway cross-talks with Forkhead box M1 (FoxM1), an oncogenic transcription factor that is regulated by levels of O-GlcNAc and OGT (middle). Inflammatory responses to genotoxic stress induce activation of NF-κB that can undergo O-GlcNAcylation to mediate genes in the immune response (left). The loss of p53 activates NF-κB to increase aerobic glycolysis and support tumor metabolism. Hyper O-GlcNAcylation of p53 stabilizes the tumor suppressor and decreases p53-MDM2 interaction to block proteolysis (bottom). In response to stress, p53 can induce cyclin-dependent kinase inhibitor p21 to inhibit proliferation.