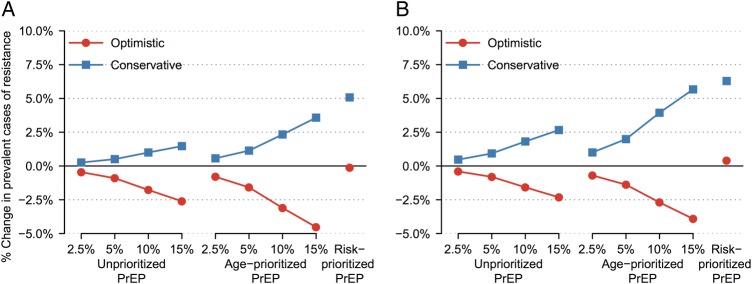
Figure 3.
Percentage change in prevalent drug-resistant cases after 10 years of pre-exposure prophylaxis (PrEP) scale-up in base-case analyses. Figures show results of (A) primary analysis, in which PrEP efficacy and drug levels persisted for 2 months after an injection, and (B) secondary analyses, in which PrEP efficacy disappeared after 2 months but drug levels persisted for 3 (optimistic) or 6 (conservative) months. Optimistic (conservative) scenario assumptions were as follows: 90% (70%) PrEP efficacy vs wild-type human immunodeficiency virus (HIV), 0%–50% relative efficacy vs rilpivirine-resistant HIV, 80% (70%) PrEP reliability, 40% (70%) cross-resistance between antiretroviral treatment and PrEP, and successful (all) PrEP injections select drug-resistant HIV after breakthrough infection.