Fig 8. Short time stability of the Drug Product in the presence of adjuvants.
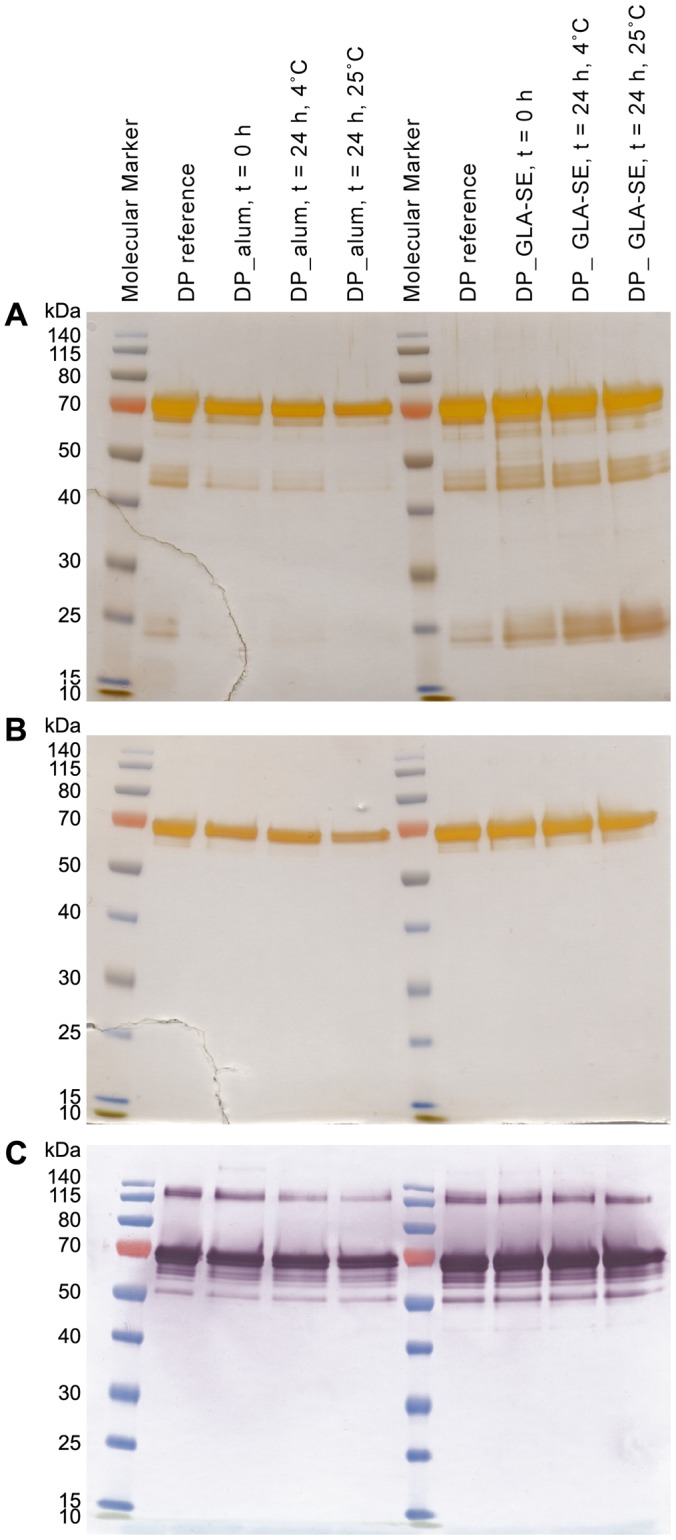
Drug Product (DP) was formulated with either Alhydrogel (alum) or GLA-SE. Formulae were broken either directly after formulation (0 hours) or after 24 hours of storage at 4°C or at 25°C. Samples were analysed with SDS-PAGE, both reduced/silver staining (panel A) and non-reduced/silver staining (panel B) and by (non-reduced) western-blot with the monoclonal antibody 4G2 (panel C).
