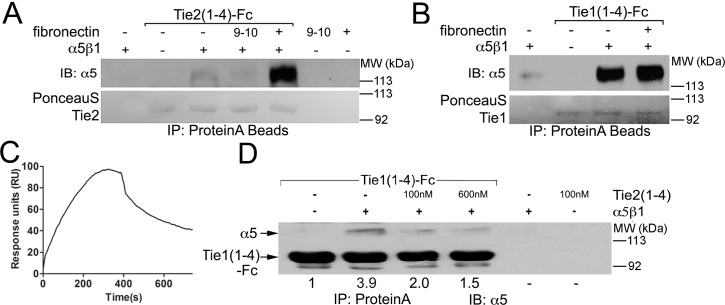
Fig 2. Direct association between Tie1 and Tie2 and integrins using purified proteins.
(A) Purified Tie2(1–4)-Fc protein (33nM) was incubated with purified α5ß1 ectodomain protein (12.5nM) in the presence or absence of either full-length or recombinant fibronectin (5μg/mL) containing only the 9th and 10th fibronectin type-III repeats (9–10). All purified components were present at the indicated concentrations in 1mL of HBST buffer and incubated overnight with 25μL ProteinA resin. Following precipitation of Tie2(1–4)-Fc with Protein-A sepharose, proteins were electrophoresed by SDS-PAGE, transferred to nitrocellulose, and Tie2 was briefly visualized with PonceauS prior to western blotting with α5-specific antibodies. The α5ß1 ectodomain readily precipitates with Tie2, although the addition of full-length fibronectin, but not recombinant, truncated fibronectin (9–10), greatly enhances the Tie2/integrin interaction. (B) As in (A) using Tie1(1–4)-Fc protein (33nM) incubated with purified α5ß1 ectodomain protein (12.5nM) in the presence or absence of full-length fibronectin (5μg/mL). Unlike the experiments shown in (A), fibronectin does not significantly enhance the Tie1/integrin interaction. (C) Surface Plasmon Resonance depiction of purified and untagged α5ß1 binding to immobilized Tie1 (Fc tagged full extracellular domain protein). Similar experiments were completed with immobilized Tie2, however no interaction was captured. (D) 33nM purified Tie1(1–4)-Fc protein was incubated with purified α5ß1 ectodomain (12.5nM) in the presence or absence of an increasing amount of Tie2(1–4) untagged protein (either 100nM or 600nM as indicated). Following precipitation of Tie1(1–4)-Fc with Protein-A sepharose, proteins were subjected to western blotting with α5-specific antibodies. α5ß1 readily precipitates with Tie1, demonstrating that the Ig and EGF repeats are sufficient for α5ß1 recognition. Furthermore, Tie2 (1–4) effectively competes with Tie1 for integrin binding, suggesting a shared binding interface of Tie1 and Tie2 on the integrin molecule. Quantified values represent a normalized ratio of α5 to Tie1(1–4)-Fc.