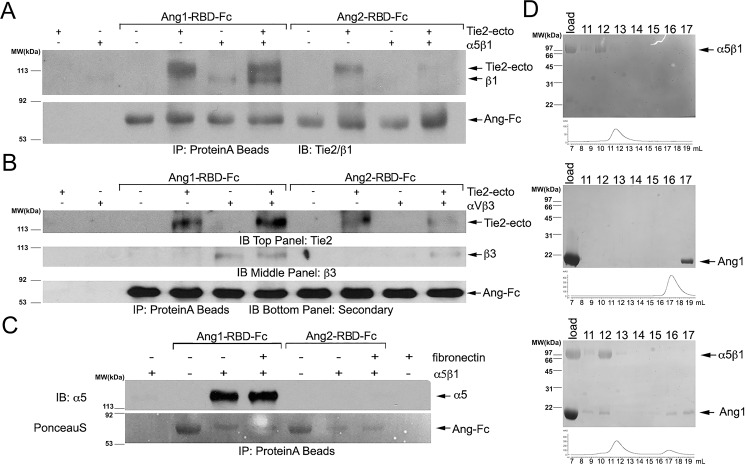
Fig 3. Direct association between the Ang-1 receptor binding domain and αVß3 and α5ß1.
(A) The Ang-1 and Ang-2 receptor binding domains were expressed and purified as Fc fusion proteins, and incubated at 50nM with purified α5ß1 (12.5nM) in the presence or absence of the Tie2 ectodomain (33nM). Precipitating proteins were visualized with Tie2 and ß1 -specific antibodies (α5 migrates at the same position as Tie2 and, therefore, α5-antibodies could not be used). The Tie2 ectodomain readily precipitates with both the Ang-1 or Ang-2 receptor binding domains, while α5ß1 only precipitates with Ang-1-RBD. (B) Similar to the experiment in (A) using purified ectodomain of αVß3 protein incubated with either Ang-1-RBD-Fc or Ang-2-RBD-Fc. Precipitating αVß3 was visualized with antibodies specific to ß3. (C) The Ang-1 and Ang-2 receptor binding domain Fc fusion proteins (50nM) were incubated with purified α5ß1 (12.5nM) in the presence or absence of human fibronectin. Following precipitation of Ang-Fc proteins with Protein-A sepharose, proteins were electrophoresed by SDS-PAGE, transferred to PVDF, and briefly visualized with PonceauS prior to western blotting with α5-specific antibodies. The α5ß1 ectodomain readily co-precipitates with Ang-1-RBD, but not with Ang-2-RBD. Fibronectin does not appear to significantly influence the Ang-1/integrin interaction. (D) Direct association between Ang-1-RBD (10μM) and α5ß1 (5μM) by size-exclusion chromatography. Indicated fractions were incubated in loading buffer with BME for 5 minutes and resolved on a 10% SDS-PAGE gel to separate proteins eluting from the column in reducible crosslinked complexes. Top panel: Purified α5ß1 ectodomain was chromatographed on a Superdex 200 column and elutes as a single peak at ~12 mL. Middle panel: Purified Ang-1-RBD elutes from a Superdex 200 as a single peak at ~17 mL. Bottom panel: 5μM α5ß1 purified protein was cross-linked to a 2 molar excess of Ang-1-RBD using DTSSP and separated on a Superdex 200 column. Under these conditions, Ang-1-RBD now elutes in a complex with α5ß1 at ~11.5 mL.