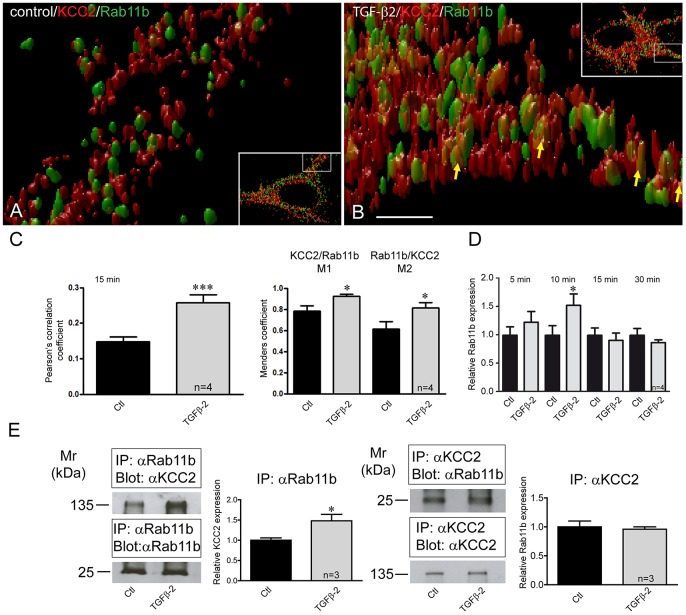
Fig. 7.
Rab11b colocalizes and interacts with KCC2 and its expression depends on TGF-β2. (A,B) 3D STED images illustrating part of dendrite of an untreated control neuron (inset in A) and a TGF-β2-treated neuron (inset in B) were acquired. High magnification images correspond to the white-boxed area of the inset. Scale bar: 500 nm. (C) Pearson's correlation coefficient and Manders' coefficients for KCC2 and Rab11b were calculated and were significantly increased in neurons treated with TGF-β2 for 15 min. ***P=0.0005 for the Pearson's correlation coefficient, and *P=0.015 and *P=0.025 for M1 and M2, respectively (unpaired Student's t-test). (D) DIV12 cultured mouse hippocampal neurons were treated for 5–30 min with 2 ng/ml TGF-β2, followed by quantitative RT-PCR analysis for Rab11b expression. *P=0.033 for relative Rab11b expression after TGF-β2 application (unpaired Student's t-test). (E) Interaction of KCC2 with Rab11b in controls and TGF-β2-treated DIV12 hippocampal neurons. Antibodies against Rab11b were able to immunoprecipitate (IP) KCC2 expressed in control hippocampal neurons, as detected by immunoblotting with KCC2 antibody. Antibodies against KCC2 were also able to immunoprecipitate Rab11b expressed in control hippocampal neurons, as detected by immunoblotting with Rab11b antibody. The ratio of ∼135 kDa KCC2:input Rab11b, ∼25 kDa Rab11b:input KCC2 in untreated (Ctl) and TGF-β2-treated cultures were then determined and presented relative to values for controls (set at 1). The amount of co-immunoprecipitated KCC2 was significantly increased in TGF-β2-treated neurons, compared to the untreated controls (*P=0.04, unpaired Student's t-test). Data are given as mean±s.e.m. from three or four independent experiments as indicated.