1. Introduction
Alcohol use typically begins early in adolescence (Faden, 2006). This use differs from that of adults, with adolescents drinking on average twice as much per drinking episode than adults (SAMHSA, 2008). This intake sometimes reaches high levels, with 23.7% of high school seniors in the United states reporting consumption of 5+ drinks (Johnston et al., 2013), >10% reporting consumption of 10+ drinks and >5% reporting 15+ drink consumption (Patrick et al., 2013) per drinking episode in the past 2 weeks.
Studies in laboratory animals such as the rat have likewise revealed 2–3 fold higher ethanol consumptions during adolescence than in adulthood (Doremus et al., 2005, Hargreaves et al., 2011; Schramm-Sapyta et al., 2014; Vetter-O’Hagen et al., 2009), suggesting that the elevated ethanol intake of adolescents may have a biological component. Indeed, studies in rats have revealed a number of potential contributors to elevated adolescent intake, including notable differences between adolescents and adults in their sensitivity to the acute effects of ethanol. For instance, adolescents show social facilitation in response to low doses of ethanol that is not seen in adults (Varlinskaya & Spear, 2002) and are also more sensitivity than adults to ethanol’s rewarding effects (e.g., Pautassi et al, 2008); these effects may make ethanol particularly desirable for adolescents. On the other hand, adolescents are less sensitive than adults to the social impairing, motor disrupting, aversive and sedative effects of higher doses of ethanol (Anderson et al, 2010; Ramirez & Spear, 2010; Silveri & Spear, 1998; Varlinskaya & Spear, 2002) – effects of ethanol likely serving to moderate drinking. This combination of age-dependent ethanol sensitivities appears to be associated with differential rates of development of neural systems underlying these ethanol effects (see Spear, 2014, for discussion). Although comparable studies in humans are not surprisingly limited due to ethical constraints regarding administration of ethanol to youth, what findings are available provide suggestions of similar age-related ethanol sensitivities in human adolescents that may help drive the elevated consumption levels characteristic of this age period (for review, see Spear, under revision).
The early initiation and relatively high use levels of alcohol by adolescents could potentially disrupt maturational changes occurring in the brain at this time in regions such as those critical for cognitive control (Casey et al., 2008), motivational responding and the processing of rewarding, arousing, aversive, social and emotional stimuli (Blakemore, 2012; Ernst & Fudge, 2008). Indeed, early onset of drinking has been found to be predictive of alcohol-related problems later in life (Grant & Dawson, 1997; Palmer et al., 2009), with those engaging in even episodic heavy drinking at an early age being more likely to develop alcohol use disorders (Bonomo et al., 2004; Hingson et al., 2006; Grant et al., 2001) and to experience a number of long-lasting adverse psychosocial consequences (Wells et al., 2004). Although these associations are not necessarily causal (Black et al., 2015; Edwards et al., 2014), evidence is emerging that binge patterns of adolescent drinking that bring blood alcohol levels to 80 mg/dl and higher can be disruptive to the developing adolescent brain (Bava & Tapert, 2010; Silveri, 2012; Spear, 2015).
Given developmental changes in alcohol-sensitive brain circuits implicated in responsiveness to social and emotional stimuli (e.g., Blakemore, 2012; Spear, 2011), it is not surprising, therefore, that anxiety, depression, and enhanced stress reactivity have often been associated with alcohol-dependency in adolescents (Martin et al., 2000). Alcohol-related problems are often evident in anxious adolescents, with social anxiety disorder sometimes noted as a particular vulnerability factor (Black et al., 2015; Buckner et al., 2006), however, causal relationships are still not fully established (e.g., Morris et al., 2005). For instance, social anxiety has been reported to often precede alcohol use disorders (Buckner et al., 2008). Sex differences are sometimes apparent, with anxiety disorders in early adolescence associated with later adolescent alcohol use in females but not males (Zehe et al., 2013). Conversely, there is also evidence that alcohol abuse can promote later expression of anxiety disorders (e.g., Falk et al., 2008; Kushner et al., 2000), with alcohol use disorders preceding the onset of anxiety more often in males than in females (Falk et al., 2008). Bi-directional associations between anxiety disorders and alcohol use could initiate a “feed-forward cycle”, with anxiety symptoms inducing alcohol use for its anxiolytic effects, whereas increasing use of alcohol may further enhance anxiety symptoms (see Kushner et al., 2000).
The relationship between adolescent anxiety and alcohol use disorders is even more complicated, given emerging evidence pointing to a substantial role of stress (negative life events) in both affective disorders and adolescent alcohol use (Fidalgo et al., 2008; Lopez et al., 2005; Low et al., 2008; Rutledge & Sher, 2001; Schmidt et al., 2007). Given that the developmental transition from immaturity/dependence to maturity/independence is often stressful, it is not surprising that adolescents have been reported to experience more stressors and negative life events than either children or adults (Buchanan et al., 1992). An increased prevalence of stressors during this ontogenetic period, when combined with enhanced stress reactivity relative to other ages (Dahl & Gunnar, 2009), may contribute to the onset of psychiatric problems during adolescence. Indeed, strong associations have been reported between adolescent stress and the emergence of anxiety disorders (Barrocas & Hankin, 2011; Grant et al., 2003; Oldehinkel & Bouma, 2011). Although the relationship between alcohol use and stressful life events has been shown to be quite complex (Uhart & Wand, 2009), it has been suggested that stress is most strongly associated with heavy drinking in adolescence, with this association becoming considerably weaker later in life (Aseltine & Gore, 2000).
Human studies of the consequences of underage alcohol use do not permit systematic manipulation of critical variables, due to ethical considerations that preclude administration of alcohol to young adolescents. Similarities found between human adolescents and adolescents of various mammalian species in terms of developmental history, neural, hormonal and behavioral alterations (Spear, 2000, 2011; Spear & Varlinskaya, 2010) provide reasonable justification for the use of animal models for assessments of the relationship between adolescent alcohol exposure, stressors, and anxiety. In humans, adolescence generally refers to a transitional period between youth and maturity that occurs predominantly during the second decade of life (Petersen et al., 1996). In rats, many behavioral, hormonal, physiological and neural characteristics of early to mid-adolescence are evident between postnatal days (P) 28 and 42, with later ontogenetic periods (until ~P55–65) generally more analogous to late adolescence/emerging adulthood in humans (Spear, 2000; Vetter-O’Hagen and Spear, 2012).
Using a rat model of adolescence, we have shown that, similar to human findings (e.g., Zehe et al., 2013) initially high socially anxious adolescent females, but not high socially anxious adolescent males, demonstrate enhanced sensitivity to the socially anxiolytic effects of ethanol and high levels of ethanol intake under social circumstances (Varlinskaya et al., 2015a,b). In contrast, repeated exposure to ethanol through early/mid adolescence has long-lasting detrimental consequences in males, but not females (Varlinskaya et al., 2014). These males, like their human counterparts (e.g., Falk et al., 2008), demonstrated anxiety-like behavioral alterations, indexed via significant decreases in social investigation and social preference when tested in adulthood (Varlinskaya et al., 2014). As adults, males exposed to ethanol early in adolescence also exhibited adolescent-characteristic responding to ethanol, demonstrating ethanol-induced social facilitation that is not normally evident in adulthood (e.g., see Varlinskaya & Spear, 2002). Intriguingly, similar behavioral alterations (i.e., decreases in baseline social investigation and social preference and induction of ethanol-induced social facilitation) also emerged following repeated exposure to restraint stress in adult male and female rats that were naïve to ethanol (Varlinskaya et al., 2010).
Given apparent similarities in the social consequences evident following repeated ethanol and repeated restraint, Experiment 1 of the present study was designed to assess whether the social anxiety-like behavior associated with adolescent intermittent ethanol (AIE) exposure to 3.5.g/kg given intragastrically (i.g.) every other day (P25–P45) is further exacerbated by later stressors (restraint, 90 min/day, P66–P70) in males and whether such stressor exposure might possibly precipitate expression of latent social anxiety in AIE females. Another aim of Experiment 1 was to assess the impact of AIE alone or in combination with stress on intake of a sweetened ethanol solution under social circumstances (see Table 1). The two main goals of Experiment 2 were (a) to assess whether AIE alters adaptation of the hormonal stress response to acute and repeated restraint (indexed via corticosterone) and (b) to determine the impacts of AIE and the repeated stressor on intake of the sweetened solution alone (see Table 2).
Table 1.
Timeline for Experiment 1.
| P25 – P45 | P66 – P70 | P70 | P77 | P80 – P83 | P88 – P91 |
|---|---|---|---|---|---|
| EtOH i.g. 3.5 g/kg every other day | No Restraint | SI test | SI test | Social Drinking (30 min, 10% EtOH in “supersac”) | Social Drinking (30 min, 10% EtOH in “supersac”) Trunk blood samples for BEC on P91 |
| Restraint (90 min/day) | |||||
| Water i.g. every other day | No Restraint | SI test | SI test | Social Drinking (30 min, 10% EtOH in “supersac”) | Social Drinking (30 min, 10% EtOH in “supersac”) Trunk blood samples for BEC on P91 |
| Restraint (90 min/day) |
Table 2.
Timeline for Experiment 2.
| P25 – P45 | P66 – P70 | P80 – P83 | P88 – P91 |
|---|---|---|---|
| EtOH i.g. 3.5 g/kg every other day | No Restraint | Social Drinking (30 min “supersac” only) | Social Drinking (30 min “supersac” only) |
| Restraint (90 min/day), tail blood samples for CORT on P66 and P70 | |||
| Water i.g. every other day | No Restraint | Social Drinking (30 min “supersac” only) | Social Drinking (30 min “supersac” only) |
| Restraint (90 min/day), tail blood samples for CORT on P66 and P70 |
2. Results
2.1. Effects of AIE on stress-induced social alterations and ethanol intake under social circumstances in adulthood
2.1.1. Body weight
When body weight data were assessed prior to the social interaction test on P70 and P77, no effects of adolescent exposure or stress were evident in males and females (all ps > 0.05). As expected, there were significant main effects of sex evident at P70, F(1, 56) = 540.44, p < 0.0001, as well as at P77, F(1, 56) = 555.72, p < 0.0001, with males having greater body weight than females at both ages (473.5 ± 7.5 g versus 272.9 ± 3.5 g at P70; 489.0 ± 8.1 g versus 273.8 ± 3.6 g at P77).
2.1.2. Social Investigation
When immediate consequences of restraint were assessed on P70, restraint effects were evident only in water-exposed animals [adolescent exposure × stress interaction, F(1, 56) = 5.4, p < 0.05], with restraint stress suppressing social investigation regardless of sex (Figure 1). There was also a significant early exposure × sex interaction, F(1, 56) = 13.77, p < 0.001, with AIE suppressing social investigation only in males, replicating prior findings (Varlinskaya et al., 2014).
Figure 1.
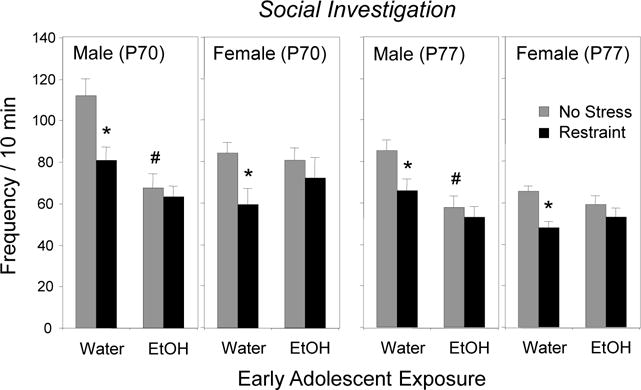
The impact of early adolescent intermittent exposure and repeated restraint on social investigation during a 10-min modified social interaction test in males and females when tested immediately (P70) or 7 days following restraint (P77). Significant effects of AIE relative to the water-exposed controls are indicated with # (p < 0.05), whereas significant effects of restraint within each exposure/sex condition are indicated with * (p < 0.05).
Delayed effects of restraint assessed a week later on P77 revealed the same patterns, with water-exposed males and females still demonstrating reductions in social investigation when tested 7 days after the last exposure to restraint [adolescent exposure × stress interaction, F(1, 56) = 4.38, p < 0.05]. AIE males, but not females, again demonstrated significant decreases in social investigation relative to their water-exposed counterparts [adolescent exposure × sex interaction, F (1, 56) = 9.46, p < 0.005].
2.1.3. Social Preference
Similar to social investigation, significant adolescent exposure × sex interactions were evident on P70, F(1, 56) = 6.15, p < 0.05, as well as on P77, F(1, 56) = 8.99, p < 0.005, with only ethanol-exposed males demonstrating significant decreases in social preference relative to their water-exposed counterparts (Figure 2). Stress effects again were seen only in water-exposed males and females, with stressor-associated decreases in social preference evident both immediately following the final restraint session [adolescent exposure × stress interaction, F(1, 56) = 4.68, p < 0.05], as well as 7 days later [adolescent exposure × stress interaction, F(1, 56) = 5.08, p < 0.05].
Figure 2.
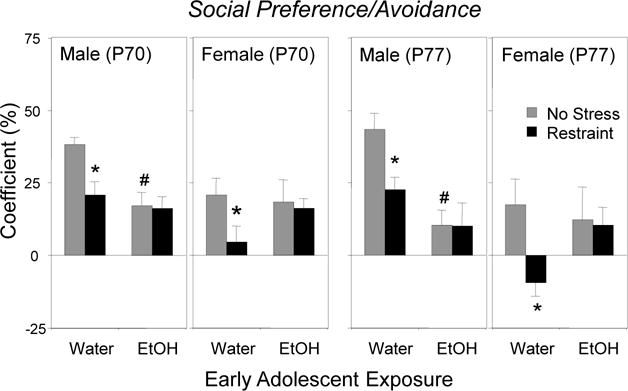
The impact of early adolescent intermittent exposure and repeated restraint on the coefficient of social preference/avoidance during a 10-min modified social interaction test in males and females when tested immediately (P70) or 7 days following restraint (P77). Significant effects of AIE relative to the water-exposed controls are indicated with # (p < 0.05), whereas significant effects of restraint within each exposure/sex condition are indicated with * (p < 0.05).
2.1.4. Overall Locomotor Activity
Overall locomotor activity within the social context (indexed via total number of crossovers) did not differ as a function of adolescent exposure, stress exposure or sex either on P70 or on P77 (all ps > 0.05; 36.3 ± 1.2 crossovers for P70 and 30.9 ± 1.3 for P77).
2.1.5. Social Drinking
Social drinking was not affected by either adolescent exposure or restraint (see Figure 3, left panel, with data collapsed across sex and test days), although the ANOVA revealed a significant interaction between sex and drinking day, F(7, 392) = 4.14, p < 0.001. Adult females ingested significantly more ethanol on a g/kg basis than males on all test days but Day 4, regardless of adolescent exposure and prior stress (Figure 3, right panel). Males gradually increased ethanol intake from Day 1 to Day 3, with intake remaining stable thereafter, whereas females demonstrated peak ethanol intake on Day 2. No sign of an “alcohol deprivation effect” emerged in males, with intake not elevated following the 4-day abstinence period (i.e., test Day 5 vs. test Day 4). In contrast, females demonstrated a modest, albeit significant increase in ethanol intake on test Day 5 relative to test Day 4.
Figure 3.
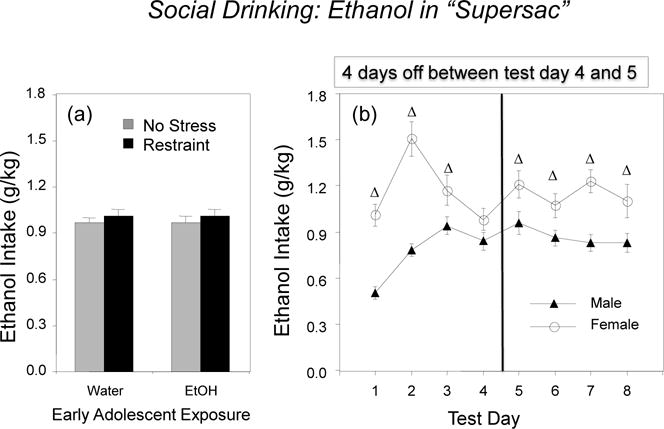
Ethanol intake under social circumstances in males and females: (a) the impact of early adolescent intermittent exposure and repeated restraint (left panel) and (b) sex differences in intake across test days (right panel). Significant effects of sex are indicated with a Δ.
On the last test day, ethanol intake and BECs did not differ as a function of adolescent exposure or prior stress. Females were found to consume significantly more ethanol than males (1.10 ± 0.11 g/kg versus 0.83 ± 0.06 g/kg, respectively), although BECs did not differ significantly as a function of sex (females: 57.8 ± 6.9 mg/dl, males: 50.2 ± 5.2 mg/dl).
When the adolescent-typical behavior of play fighting was assessed and analyzed during the social drinking session on the last test day, the ANOVA revealed a significant adolescent exposure × stress condition × sex interaction, F(1, 56) = 4.24, p < 0.05 (see left panels of Figure 4). Among non-stressed animals, AIE altered play fighting during social drinking in a sex-dependent manner, increasing play fighting in males but decreasing play in females under these same social drinking conditions. Among water-exposed males, stress increased play fighting during social drinking relative to their counterparts that were not stressed.
Figure 4.
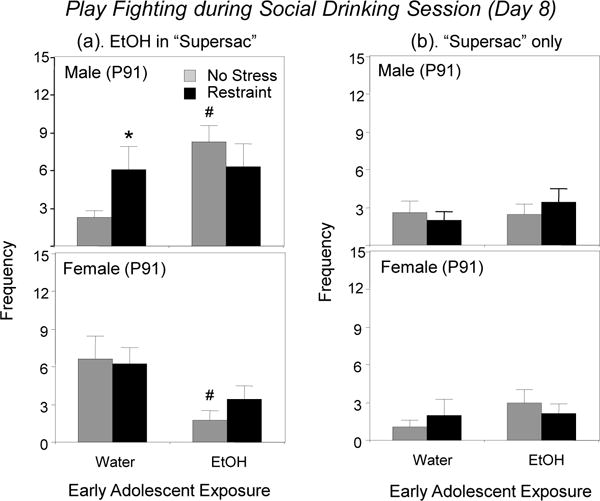
The impact of early adolescent intermittent exposure and repeated restraint on the adolescent-typical behavior of play fighting during social drinking of (a) ethanol in “supersac” (left panels) or (b) “supersac” only (right panels) on Day 8 in males and females. Significant effects of AIE relative to water-exposed controls within each sex are indicated with # (p < 0.05), whereas significant effects of restraint within each exposure/sex condition are indicated with * (p < 0.05).
2.2. Effects of AIE on stress-induced corticosterone levels and intake of “supersac” under social circumstances in adulthood
2.2.1. Restraint-induced Corticosterone Levels
Corticosterone (CORT) levels were assessed from tail bloods taken at 30 min into the 90 min restraining period, given that adult males and females demonstrate peak CORT levels at approximately this time (Doremus-Fitzwater et al., 2009). The overall 2 (adolescent exposure) × 2 (sex) × 2 (day) mixed factor ANOVA revealed a significant main effect of sex, F(1, 28) = 216.93, p < 0.0001, tempered by a significant adolescent exposure × day interaction, F(1, 28) = 13.37, p < 0.01. As expected, females had significantly greater CORT levels 30 min following the onset of the stressor (976.8 ± 28.0 ng/ml) than males (380.4 ± 26.5 ng/ml). Separate ANOVAs were conducted on data within each sex to further explore the impact of AIE on the CORT response across days. In the analysis of the CORT data in males, a significant adolescent exposure × day interaction emerged, F(1, 14) = 5.80, p < 0.05. On the first day of restraint stress, both ethanol- and water-exposed males exhibited comparable CORT levels 30 min into stressor, whereas on the last day significant habituation of the CORT response at this time point was evident only in water-exposed males (see Figure 5). Similar patterns of CORT changes over days were evident in females as well [exposure × day interaction, F(1, 14) = 4.70, p < 0.05], with water-exposed females, but not their ethanol-exposed counterparts, demonstrating habituation of the CORT levels on day 5 versus day 1.
Figure 5.
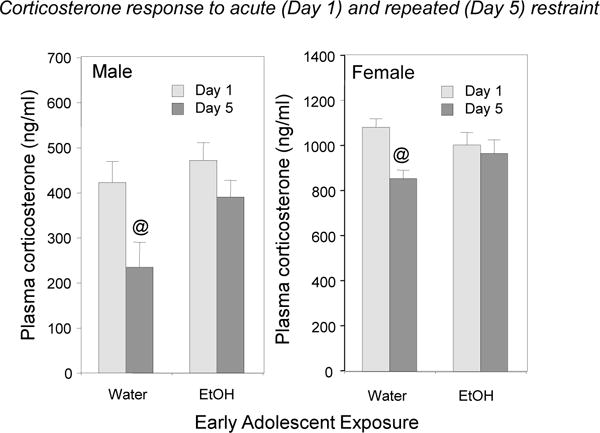
Corticosterone responses to acute (Day 1) and repeated (Day 5) restraint in males and females: the impact of early AIE. Significant habituation indexed via significant decreases in CORT levels on Day 5 relative to Day 1 within adolescent exposure/sex conditions is marked with @ (p < 0.05).
2.2.2. Social Drinking
The 2 (adolescent exposure: water, ethanol) × 2 (stress: no, restraint) × 2 (sex) × 8 (drinking day) mixed factor ANOVA revealed a significant four-way interaction, F(7, 392) = 2.19, p < 0.05 (see Figure 6). In general, females ingested more “supersac” on a ml/kg basis that males regardless of adolescent exposure and stress condition on all test days, with this sex difference being the smallest on Day 1. Males showed a significant, though moderate increase in “supersac” intake from Day 1 to Day 2, whereas females more than doubled their intake on Day 2 relative to Day 1 and maintained intake at relatively high levels through the entire drinking period. Intake of “supersac” was enhanced by a 4-day off period in females regardless of adolescent exposure and stress condition, and in all male groups but water-exposed non-stressed males. In females, intake of “supersac” was not affected by either adolescent exposure or stress condition. In contrast, repeated restraint decreased “supersac” intake in ethanol-exposed males relative to their non-stressed counterparts on test Days 4 – 8, while increasing it in water-exposed males on Day 5 (see Figure 6).
Figure 6.
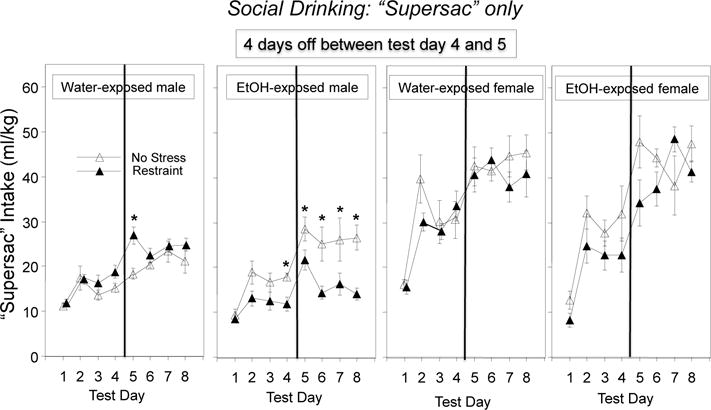
“Supersac” intake under social circumstances in males and females: the impact of early AIE exposure and repeated restraint. Significant stress-induced changes within test day are indicated for each adolescent exposure/sex condition with * (p < 0.05).
The ANOVA of play fighting during the social drinking session with “supersac” on the last test day revealed no significant main effects or interactions. Thus, the level of play fighting when ingesting sweet solution under social circumstances was not affected by AIE or stress in neither males nor females (see Figure 4, right panels).
3. Discussion
As in our previous study (Varlinskaya et al., 2014), the effects of AIE on social anxiety-like behavioral alterations in adulthood differed drastically in males and females, with only males demonstrating these alterations in a modified social interaction test. These sex-dependent findings are reminiscent of sex differences reported for humans, with alcohol use promoting later expression of anxiety disorders more frequently in males than in females (Falk et al., 2008). Similar to their human counterparts, non-stressed males exposed to ethanol as adolescents demonstrated anxiety-like alterations evident through significant reductions in social investigation and social preference relative to their water-exposed counterparts.
High levels of social investigation and social preference demonstrated by water-exposed control animals were substantially reduced by exposure to repeated stress in adult males and females, with these effects seen both immediately post-stressor (at P70) and one week thereafter (P77). The former results are reminiscent of our previous findings (Doremus-Fitzwater et al., 2009; Varlinskaya et al., 2010) where otherwise non-manipulated adolescent and adult males and females tested immediately post-stressor demonstrated consequences of repeated restraint similar to those evident in water-exposed rats in the present study. In both those studies and the current experiment, social preference and/or social investigation were significantly decreased by prior exposure to the stressor, suggesting that these two social measures are extremely sensitive to anxiogenic manipulations under normal circumstances.
Adolescent exposure to ethanol altered the social response normally seen to repeated restraint, with these animals not showing the stress-associated decrease in social interactions seen in adults that were not exposed to ethanol in adolescence. While this could reflect a floor effect in AIE males (given that they exhibited baseline reductions in social investigation and social preference even when not stressed), this was not the case with females, given that AIE alone did not suppress these social measures in female subjects. An alternative interpretation of these findings is that the AIE exposure may have attenuated later stress reactivity in these animals. The supersaccharin intake anhedonia data (in males) and the corticosterone data in animals of both sexes provide compelling evidence, however, that AIE-exposed rats are reactive to stressors in adulthood. For instance, in terms of the CORT data, whereas adults of both sexes that only received water exposure during adolescence demonstrated adult-typical habituation of the CORT response from Day 1 to Day 5, adult male and female subjects exposed to ethanol as adolescents demonstrated no evidence of habituation across days, suggesting likely dysregulation of the HPA axis associated with early AIE in both males and females. Although adult exposure groups were not included in this experiment, it is possible that the observed alterations in CORT response habituation may be especially pronounced after adolescent exposure, given that comparable ethanol exposure in adulthood was not found to affect habituation of the CORT response to a stressor (Przybycien-Szymanska et al., 2011). Taken together, these findings may have translational relevance; given data from human studies that chronic alcohol produces dysregulation of the HPA axis and, therefore, induces a persistent impairment in the ability to adequately respond to stress (Sher, 2007).
The lack of habituation in adult animals after AIE is reminiscent of the adolescent-typical CORT response to restraint. Whereas prior work has shown that adult rats habituate to repeated restraint, as evident by notably diminished levels of CORT on the last relative to first day of stressor exposure, young adolescent animals show no habituation of the CORT response to a repeated homotypic stressor (Eiland & Romeo, 2013; Doremus-Fitzwater et al., 2009; Romeo et al., 2006; Romeo, 2010). Therefore, repeated exposure to ethanol early in adolescence appears to preserve adolescent-characteristic hormonal responding to a stressor, with this effect evident in both males and females tested in adulthood. It is likely that repeated ethanol exposure prevents age-appropriate maturation of the HPA axis that normally occurs during adolescence and is associated, to some extent, with pubertal maturation (McCormick & Mathews, 2007; McCormick et al., 2010; Romeo, 2010). These findings extend to a hormonal measure other work reporting an AIE-induced retention of adolescent-typical phenotypes into adulthood based on behavioral, electrophysiological, and neuroanatomical data (see Spear & Swartzwelder, 2014, for review and references).
Neither AIE nor stress had any effects on ethanol intake in a social drinking paradigm, with females drinking more than males regardless of adolescent ethanol and adult stress exposure conditions. The elevated intake of adult females relative to adult males observed in the present study is consistent with other intake data in adult rodents, in which a similar sex difference has been reported under a number of intake testing conditions (Broadwater et al., 2013; Cailhol & Mormede, 2001; Chester et al, 2006; Doremus et al, 2005; Varlinskaya et al., 2015a; Vetter-O’Hagen et al., 2009). Results of studies assessing effects of adolescent exposure to ethanol in rats on later intake of ethanol are mixed, with studies reporting increases in ethanol intake following adolescent ethanol exposure (Pandy et al., 2015; Pascual et al., 2009; Siciliano & Smith, 2001) contrasting with others that found no increases in later intake (Toliver & Samson, 1991; Vetter et al., 2007) that were independent of solution acceptance due to its familiarity (Broadwater et al., 2013).
In humans, high levels of drinking, especially early in adolescence, increase later alcohol use and problems associated with that use (Grant & Dawson, 1997; Dawson et al., 2008). However, we did not observe any increases in ethanol intake under social circumstances following AIE alone or in combination with repeated restraint. These discrepancies between human and animal data may be related to a number of different factors. Among these factors, the forced nature of adolescent exposure to ethanol may play a substantial role (Ufer et al., 1999), given that experimenter-administered ethanol can mimic some but not all aspects of adolescent ethanol exposure. Although the ideal route of adolescent exposure arguably would be through ethanol drinking, the i.g. route was chosen in this study not only to provide consistent g/kg exposure to ethanol across animals, but also to achieve BECs well into the binge range.
It has been shown previously that adolescent rats tested under familiar, non-anxiogenic circumstances demonstrate increases in social behavior following acute i.p. administration of relatively low doses (0.5–0.75 g/kg) of ethanol, an ethanol-induced facilitation of social behavior that is predominantly characterized by an increase in play fighting and is not normally seen in adults (Spear and Varlinskaya, 2005; Trezza et al., 2009; Varlinskaya and Spear, 2002, 2006, 2007; Willey et al., 2009). Such low-dose ethanol-induced social facilitation has been shown to be expressed in adulthood following restraint stress in both males and females (Varlinskaya et al., 2010). The current data demonstrate similar increases in play fighting following voluntary ethanol ingestion in stressed water-exposed males, but not females, thereby providing evidence for sex differences in sensitivity to the socially stimulating effects of ethanol when it is ingested voluntarily. These sex differences may be related at least in part to initially high levels of play fighting evident in non-stressed water-exposed females, suggesting that the chronic perturbation of water administration during adolescence in itself may be sufficient to increase sensitivity of adult females to ethanol-induced social facilitation. These high levels of play fighting were not evident, however, in non-stressed females given AIE. This significant difference could perhaps be a function of increased sensitivity of AIE females to ethanol-induced social suppression following voluntary ethanol ingestion. Yet, previously we have observed that sensitivity to ethanol-induced social suppression following i.p. ethanol challenge did not differ between AIE females and their control counterparts (Varlinskaya et al., 2014), weakening this suggestion.
In Experiment 1 of the present study, adult males that demonstrated increases in play fighting on Day 8 during the social drinking session (stressed water-exposed and ethanol-exposed males) ingested about 0.83 g/kg ethanol and demonstrated mean BECs around 50 mg/dl (see 2.1.5. Social Drinking). These BECs are in the range of those produced by ethanol doses inducing social facilitation after i.p. administration (i.e., ~ 40 to 80 mg/dl; see Varlinskaya & Spear, 2002, 2015). It is tempting to speculate from these findings that AIE males, as well as stressed water-exposed males may modulate and limit their ethanol intake to achieve ethanol levels optimal for experiencing the socially facilitating effects of ethanol.
Social facilitation seen in adolescent animals following acute ethanol administration is seemingly mediated, at least in part, through ethanol-induced release of endogenous ligands for the mu-opioid receptor (MOR) or an ethanol-associated enhancement of sensitivity of these receptors to their endogenous ligands, since the facilitation of play fighting by low doses of ethanol can be attenuated by the nonselective as well as by selective mu opioid antagonists (Varlinskaya and Spear, 2009). Indeed, the MOR system is implicated in modulation of play behavior, with selective MOR agonists increasing play fighting in young adolescent males and antagonists suppressing this form of social behavior (see Trezza et al., 2010 for references and review). While the endogenous MOR system plays a considerable role in facilitation of play fighting by ethanol (Trezza et al., 2009; Varlinskaya & Spear, 2009), other neural systems are implicated in ethanol-associated modulation of play fighting as well. For instance, social behavior during adolescence can be facilitated by indirect cannabinoid agonists (Trezza & Vanderschuren, 2008a, 2008b), whereas CB1 receptor antagonists are able to diminish ethanol-induced facilitation of play behavior during early adolescence (Trezza et al., 2009). Play fighting in adolescent rats is also under inhibitory control of the NMDA system, with NMDA antagonists facilitating play fighting at low doses, but suppressing social behavior at higher doses (Siviy et al., 1995) – biphasic effects on play fighting similar to those induced by ethanol (Varlinskaya & Spear, 2002, 2006). The NR2B subunit of the NMDA receptor may play a particularly important role, given that a selective NR2B antagonist, ifenprodil, was found to facilitate play fighting in a manner similar to that produced by low doses of less selective NMDA antagonists as well as ethanol (Morales et al., 2013). It seems reasonable to suggest that some of the neural systems that are involved in ethanol-induced social facilitation during adolescence might play a similar role in AIE males as well as in stressed water-exposed males. If this was the case, pharmacological manipulations that have been shown to produce social facilitation in adolescents, but not in adults, should likewise be effective in terms of their socially activating effects in adult males exposed to ethanol as adolescents or to repeated stress in adulthood, with no activating effects evident in their control counterparts.
In contrast to the insensitivity of ethanol intake to adolescent exposure or stress condition (Experiment 1), stressor exposure in combination with AIE sex-dependently suppressed intake of the sweet solution used as a vehicle for ethanol when it was given alone (Experiment 2). In males, but not females, the combination of AIE and later repeated restraint substantially decreased “supersac” intake relative to control males and males given either ethanol exposure or restraint stress alone, with only five days of stressor exposure being sufficient for producing decreases in “supersac” intake. Suppressed intake of sweet solutions has been frequently used as an index of anhedonia (1997 Anisman & Matheson, 2005; Henningsen et al., 2009; Moreau et al., 1995; Willner, 1997). Anhedonia is one of the core symptoms of depression (Heshmati & Russo, 2015), and has been modeled in studies with (primarily male) rodents using exposure to unpredictable variable stressors (heterotypic stressors) applied for an extended period of time (see Wilner, 2005 for references). Although such studies have rarely used animals of both sexes, there is some evidence that females are less sensitive to stress-associated decreases in sucrose preference than their male counterparts. That is, whereas sucrose preference was often reported to be decreased to some extent in rats of both sexes, these reductions were notably more pronounced in males than females (Dalla et al., 2005, 2008; Kamper et al., 2009). Similarly, when stress-associated anhedonia was assessed in adult animals following maternal deprivation or chronic unpredictable stress in adulthood, male subjects demonstrated severe to moderate anhedonia, respectively, whereas no signs of anhedonia were evident in stressed females relative to their non-stressed controls (Bai et al., 2014).
The results of Experiment 2 demonstrate that relatively short repeated exposure to restraint (5 days) was not sufficient to produce anhedonia in either males or females under control conditions. This is in agreement with findings that reported no effects of repeated restraint (1 hr, 14 days) on sucrose preference in most experimental subjects, with only 18% of stressed male rats demonstrating some signs of anhedonia (Poulin et al., 2014), suggesting that there may be individual differences in pre-disposition to exhibit anhedonia to a relatively mild homotopic stressor. Indeed, anhedonia indexed by decreased intake of “supersac” was evident in the present study only in males that were exposed to ethanol during adolescence and restrained as adults. These results are consistent with the suggestion that early AIE may make males more vulnerable to stress-precipitated depression-like alterations, with females being less vulnerable to this effect. Alternatively, it is possible that intake of sweet solutions may not serve as an appropriate behavioral index of depressive-like behavior in females (see Franceschelli et al., 2014 for references and review), since they drink more sucrose than males under normal conditions and show marked increases in their intake over time (Dalla et al., 2005; Pitychoutis et al., 2012, see also Figure 6). Clearly, more studies are needed to further explore possible female vulnerability to stress-associated depressive-like behavioral alterations after adolescent ethanol exposure.
The results of this and our previous study (Varlinskaya et al., 2014) demonstrate pronounced sex differences in vulnerability to the harmful effects of adolescent ethanol exposure alone or in combination with stressor exposure later in life, with males being more vulnerable to these effects than their female counterparts. One possible contributor to the apparent resistance of females is that neuroactive steroids may serve to protect them from ethanol-associated alterations within brain systems implicated in anxiety- and depression-like behavior. Indeed, levels of progesterone-derived neurosteroids are higher in females than males (Corpechot et al., 1993; Torres et al., 2001), and these neurosteroids have been shown to have anxiolytic and antidepressant effects in a number of paradigms (Bitran et al., 1991; Brot et al., 1995; Eser et al., 2008; Frye & Walf, 2004; Khisti et al., 2000; Molina-Hernandez et al., 2005). Therefore, high levels of endogenous anxiolytic and antidepressant substances in females may play a role in protecting against the induction of both AIE-associated social anxiety-like behaviors as well as stress-induced anhedonia in adulthood.
In summary, the results of the present study confirm a particular vulnerability of young adolescent males to long-lasting adverse effects of repeated ethanol. In males, exposure to ethanol early in adolescence produces social anxiety-like behavioral alterations and preservation of adolescent-like sensitivity to ethanol-induced social facilitation evident following forced ethanol administration (Varlinskaya et al., 2014), as well as during voluntary ingestion of ethanol under social circumstances. Males exposed to ethanol early in adolescence also demonstrate enhanced vulnerability to consequences of adult stress: the combination of early AIE with later mild stress serves as a risk factor for the development of depressive-like behavioral alteration in males during adulthood. In humans, high rates of co-morbidity between alcohol use, anxiety and depression disorders have been reported in both adolescents and adults (Armstrong & Costello, 2002; Kessler et al., 1997; Marmorstein, 2009), with repeated occurrence of negative life events increasing vulnerability to depressive and substance use disorders (Brady & Sinha, 2005). The results of the present animal study directly demonstrate a strong association between early alcohol exposure, anxiety, stress and depression, providing a solid background for experimental research examining the mechanisms contributing to co-occurring alcohol use, anxiety and depression disorders, at least in males.
In contrast to AIE males, AIE females demonstrated no anxiety-like social alterations and did not show signs of anhedonia following repeated restraint, although both they and their male counterparts did retain into adulthood the immature, adolescent-typical pattern of CORT response to repeated versus acute stressors, suggesting an AIE-associated persisting dysregulation of the HPA axis reminiscent of that reported in humans following chronic alcohol (Sher, 2007). Given these findings, it is possible that different approaches might be needed for female subjects. These approaches could include not only higher ethanol exposure doses and different exposure regimens, but also perhaps pre-screening to determine whether detrimental effects of ethanol during adolescence or stress in adulthood may vary among females with different baseline levels of social anxiety-like behavior that potentially may differ in level of protection provided by endogenous anxiolytics.
4. Experimental Procedure
4.1. Subjects
Males and female Sprague Dawley rats bred and reared in our colony at Binghamton University were used (Experiment 1: n=64 experimental subjects plus 64 social partners; Experiment 2: n=64 subjects). All animals were housed in a temperature-controlled (22°C) vivarium maintained on a 14-/10-hr light/dark cycle (lights on at 07:00 hr) with continuous home cage ad libitum access to food (Purina Rat Chow, Lowell, MA) and water throughout these studies. Litters were culled to 8 (4 male and 4 female) pups on P1 and housed with their mothers in standard maternity cages with pine shavings as bedding material. Pups from four litters born the same day were weaned on P21, with one animal from each litter placed in each standard plastic cage of same-sex non-littermates (n=4/cage). In all respects, maintenance and treatment of the animals were in accord with guidelines for animal care established by the National Institutes of Health, using protocols approved by the Binghamton University Institutional Animal Care and Use Committee.
4.2. Experimental Design
The design for each of the two experiments was a 2 (adolescent exposure: water, ethanol) × 2 (sex) × 2 (stress: no, restraint) factorial, with 8 animals placed into each of the 8 experimental groups. Animals assigned to the same adolescent exposure and adulthood stressor condition were housed together. Animals were assigned semi-randomly to the pre-test conditions, with the constraint that no more than one subject of a given sex from a given litter was assigned to a particular pre-test condition (see Holson and Pearce, 1992; Zorrilla, 1997). Experiment 1 of the present study was designed to assess the effects of AIE and repeated restraint on (a) social anxiety-like behavior and (b) intake of a sweetened ethanol solution under social circumstances. The two main aims of Experiment 2 were to assess (a) whether AIE alters adaptation of the hormonal stress response to acute and repeated restraint (indexed via CORT) and (b) to determine the impacts of AIE and the repeated stressor on intake of the sweetened solution alone. Timelines for Experiments 1 and 2 are presented in Tables 1 and 2, respectively.
4.3. Intermittent Ethanol Exposure
In Experiments 1 and 2, animals were exposed to ethanol i.g. (3.5 g/kg, 25% solution in tap water) every other day (11 exposures) during early-mid adolescence (P25–P45). Controls were given an isovolumetric amount of tap water by gavage on these exposure days.
4.4. Stressor Procedure
In each of the two experiments, beginning at P66, animals from the repeated stress group were removed from their home cage between 1000 – 1200 hr and then restrained in plastic flat-bottom restrainers (6.35 cm diameter × 15.24 cm length for females and 8.57 cm diameter × 21.51 cm length for males) for 90 min in a novel holding cage. This restraint procedure was conducted daily for 5 days (i.e., P.66–70). Animals assigned to the no stress condition were not manipulated during this 5-day stressor phase (Doremus-Fitzwater et al, 2009). In Experiment 2, tail blood samples were collected 30 min after placing animals into the restraint tubes on Day 1 and Day 5.
4.5. Modified Social Interaction Test
In Experiment 1, social testing occurred on P70 and P77. Immediate post-stressor effects on social behavior were tested 30 min following the offset of the last stressor period (P70), whereas delayed consequences of the stressor were assessed 7 days following exposure to restraint (P77). On each of the two test days, to familiarize the test animal with the testing situation, each test subject was placed individually for 30 min in a two-compartment testing apparatus (overall dimensions: 45 × 30 × 20 cm) with an aperture (9 × 7 cm) connecting the two sides. A social partner of the same age and sex was then introduced for a 10-min test period. Partners were not pre-exposed to the test apparatus on test day, and were always unfamiliar with the experimental animal, were not socially deprived, and had not received prior i.g. exposure to ethanol or water (Varlinskaya and Spear, 2002, 2006, 2008). Partners were used two times (P70, and P77), with each experimental subject exposed to a novel partner at each test. Weight differences between test subjects and their partners were minimized as much as possible, with this weight difference not exceeding 20 g and test subjects always being heavier than their partners.
During the 10-min test session, the behavior of the animals was recorded by a video camera (Panasonic model AF-X8, Secaucus, NJ), with real time being directly recorded onto the videotape for later scoring (Easy Reader II Recorder; Telcom Research TCG 550, Burlington, Ontario). All testing procedures were conducted between 9:00 and 13:00 hr under dim light (15–20 l×). Two stress-sensitive behavioral measures, namely social investigation and the coefficient of social preference/avoidance (Doremus-Fitzwater et al., 2009; Varlinskaya et al., 2010, 2013), were scored from the video recordings and analyzed. Social investigation was defined as the sniffing of any part of the body of the partner. Social preference/avoidance was analyzed by separately measuring the number of crossovers demonstrated by the experimental subject towards as well as away from the social partner and assessed by means of a coefficient [coefficient (%) = (crossovers to the partner – crossovers away from the partner)/(total number of crosses both to and away from the partner) × 100]. Social preference was defined as positive values of the coefficient, while social avoidance was associated with negative values. Total number of crossovers was also analyzed as a rough index of general locomotor activity in this social test situation.
4.6. Social Drinking
Ethanol (Experiment 1) or “supersac” (Experiment 2) intake was assessed using a novel social drinking paradigm (Varlinskaya et al., 2015a,b) whereby animals in each cage-mate group were placed together in a novel cage for a 30-min drinking session. During this session, the animals were given free access to two bottles, both containing either 10% ethanol in “supersac” solution (3% sucrose + 0.125% saccharin, see Ji et al., 2008) or “supersac” alone (note: two bottles of the same solution were provided to reduce competition for fluid access within the group during the session). All sessions were videotaped and were conducted daily from P80 to P83 and again from P88 to P91. Between these two sets of 4-day sessions (i.e., from P84 to P87), animals were left undisturbed in their home cages. Each animal in a group was given a unique mark on its head or body with a permanent marker that allowed determination of the time spent drinking by each animal in the group from the video recording of each drinking session. Drinking behavior was characterized by the animal standing on its hind limbs and grasping the drinking tube with its forepaws, and by repetitive, regular small movements of the head up and down. This drinking behavior was distinct and readily distinguishable from brief investigation or sniffing of the drinking tube. Determination of total ethanol consumption per session (i.e., g of ethanol ingested by all cage-mates) and time each rat spent drinking, as assessed by video records of the social drinking session, was used to calculate g/kg ethanol intake for each individual animal using the following formula: (individual g/kg intake = [(g of ethanol consumed by the group/sum of time spent drinking by all cage-mates) × time for an individual animal]/body weight of the respective animal). Our previous work (Varlinskaya et al., 2015a) has revealed significant correlations between time spent drinking and BECs in individually and socially tested adult males and females (r values ranging from 0.80–0.92), therefore confirming that time spent drinking is a reliable measure for assessment of ethanol intake.
During the last drinking session, the adolescent-typical behavior of play fighting (number of playful nape attacks demonstrated by each individual animal during the 30-min drinking session) was scored and analyzed in both experiments.
4.7. Blood Ethanol Determination
In Experiment 1, for analysis of blood ethanol content, trunk blood samples were collected immediately after the last drinking session using heparinized tubes. Blood samples were then rapidly frozen and maintained at –80°C. Samples were assessed for BECs via headspace gas chromatography using a Hewlett Packard (HP) 5890 series II Gas Chromatograph (Wilmington, DE). At the time of assay, blood samples were thawed and 25-μl aliquots were placed in airtight vials. Vials were placed in a HP 7694E Auto-Sampler, which heated each individual vial for 8 min and then extracted and injected a 1.0 ml sample of the gas headspace into the chromatograph. Ethanol concentrations in each sample were determined using HP Chemstation software, which compares the peak area under the curve in each sample with those of standard curves derived from reference standard solutions.
4.7. Corticosterone Determination
In Experiment 2, 30 min after placement into the restrainers on days 1 and 5, tail blood samples were collected using heparinized tubes. Samples were then centrifugated at 2°C for 20 minutes at 3000 rpm, and the plasma frozen and maintained in a −80°C freezer until the time of assay. Plasma corticosterone (CORT) levels were analyzed by radioimmunoassay (RIA) using RIA kits obtained from INCBiomedicals, Inc. (Orangeburg, NY).
4.8. Data Analyses
Social drinking in both experiments (Experiment 1: intake of ethanol in g/kg; Experiment 2: intake of “supersac” in ml/kg) was analyzed using a 2 (adolescent exposure: water, ethanol) × 2 (stress: no, restraint) × 2 (sex) × 8 (drinking day) ANOVA, with drinking day treated as a repeated measure. BECs (Experiment 1) and play fighting (both experiments) demonstrated by experimental subjects on the last drinking day were analyzed using 2 (adolescent exposure: water, ethanol) × 2 (stress: no, restraint) × 2 (sex) ANOVAs. In Experiment 1, body weight, as well as social investigation and social preference during the social interaction testing on P70 and P77 were analyzed using separate 2 (adolescent exposure: water, ethanol) × 2 (stress: no, restraint) × 2 (sex) analyses of variance (ANOVAs). In Experiment 2, CORT levels were assessed using a 2 (adolescent exposure) × 2 (sex) × 2 (day) mixed factor ANOVA. To avoid inflating the possibility of type II errors on tests with at least three factors (Carmer & Smanson, 1973), Fisher’s planned pairwise comparisons were used to explore significant effects and interactions.
Highlights.
Early adolescent ethanol exposure (AIE) produced social anxiety in males only.
Repeated restraint had social impairing effects in both males and females.
AIE curbed sensitivity to the social impairing effects of repeated restraint.
AIE eliminated corticosterone habituation to repeated restraint in both sexes.
The combination of AIE and stress induced anhedonia only in males.
Acknowledgments
The research presented in this paper was supported by NIH grant U01 AA019972.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson RI, Varlinskaya EI, Spear LP. Ethanol-induced conditioned taste aversion in male sprague-dawley rats: impact of age and stress. Alcohol Clin Exp Res. 2010;34:2106–15. doi: 10.1111/j.1530-0277.2010.01307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisman H, Matheson K. Stress, depression, and anhedonia: caveats concerning animal models. Neurosci Biobehav Rev. 2005;29:525–46. doi: 10.1016/j.neubiorev.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Armstrong TD, Costello EJ. Community studies on adolescent substance use, abuse, or dependence and psychiatric comorbidity. J Consult Clin Psychol. 2002;70:1224–39. doi: 10.1037//0022-006x.70.6.1224. [DOI] [PubMed] [Google Scholar]
- Aseltine RH, Jr, Gore SL. The variable effects of stress on alcohol use from adolescence to early adulthood. Subst Use Misuse. 2000;35:643–68. doi: 10.3109/10826080009148415. [DOI] [PubMed] [Google Scholar]
- Bai M, Zhang L, Zhu X, Zhang Y, Zhang S, Xue L. Comparison of depressive behaviors induced by three stress paradigms in rats. Physiol Behav. 2014;131:81–6. doi: 10.1016/j.physbeh.2014.04.019. [DOI] [PubMed] [Google Scholar]
- Barrocas AL, Hankin BL. Developmental pathways to depressive symptoms in adolescence: a multi-wave prospective study of negative emotionality, stressors, and anxiety. Journal of abnormal child psychology. 2011;39:489–500. doi: 10.1007/s10802-010-9482-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava S, Tapert SF. Adolescent brain development and the risk for alcohol and other drug problems. Neuropsychology review. 2010;20:398–413. doi: 10.1007/s11065-010-9146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitran D, Hilvers RJ, Kellogg CK. Anxiolytic effects of 3 alpha-hydroxy-5 alpha[beta]-pregnan-20-one: endogenous metabolites of progesterone that are active at the GABAA receptor. Brain research. 1991;561:157–61. doi: 10.1016/0006-8993(91)90761-j. [DOI] [PubMed] [Google Scholar]
- Black JJ, Clark DB, Martin CS, Kim KH, Blaze TJ, Creswell KG, Chung T. Course of alcohol symptoms and social anxiety disorder from adolescence to young adulthood. Alcohol Clin Exp Res. 2015;39:1008–15. doi: 10.1111/acer.12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ. Development of the social brain in adolescence. Journal of the Royal Society of Medicine. 2012;105:111–6. doi: 10.1258/jrsm.2011.110221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonomo YA, Bowes G, Coffey C, Carlin JB, Patton GC. Teenage drinking and the onset of alcohol dependence: a cohort study over seven years. Addiction. 2004;99:1520–8. doi: 10.1111/j.1360-0443.2004.00846.x. [DOI] [PubMed] [Google Scholar]
- Brady KT, Sinha R. Co-occurring mental and substance use disorders: the neurobiological effects of chronic stress. Am J Psychiatry. 2005;162:1483–93. doi: 10.1176/appi.ajp.162.8.1483. [DOI] [PubMed] [Google Scholar]
- Broadwater M, Varlinskaya EI, Spear LP. Effects of voluntary access to sweetened ethanol during adolescence on intake in adulthood. Alcohol Clin Exp Res. 2013;37:1048–55. doi: 10.1111/acer.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brot MD, Koob GF, Britton KT. Anxiolytic effects of steroid hormones during the estrous cycle. Interactions with ethanol. Recent developments in alcoholism: an official publication of the American Medical Society on Alcoholism, the Research Society on Alcoholism, and the National Council on Alcoholism. 1995;12:243–59. doi: 10.1007/0-306-47138-8_16. [DOI] [PubMed] [Google Scholar]
- Buchanan CM, Eccles JS, Becker JB. Are adolescents the victims of raging hormones: evidence for activational effects of hormones on moods and behavior at adolescence. Psychological bulletin. 1992;111:62–107. doi: 10.1037/0033-2909.111.1.62. [DOI] [PubMed] [Google Scholar]
- Buckner JD, Eggleston AM, Schmidt NB. Social anxiety and problematic alcohol consumption: the mediating role of drinking motives and situations. Behavior therapy. 2006;37:381–91. doi: 10.1016/j.beth.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Buckner JD, Schmidt NB, Lang AR, Small JW, Schlauch RC, Lewinsohn PM. Specificity of social anxiety disorder as a risk factor for alcohol and cannabis dependence. Journal of psychiatric research. 2008;42:230–9. doi: 10.1016/j.jpsychires.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cailhol S, Mormede P. Sex and strain differences in ethanol drinking: effects of gonadectomy. Alcohol Clin Exp Res. 2001;25:594–9. [PubMed] [Google Scholar]
- Carmer SG, Swanson MR. An evaluation of ten pairwise multiple comparison procedures by Monte Carlo methods. Journal of the American Statistical Association. 1973;68:66–74. [Google Scholar]
- Casey BJ, Getz S, Galvan A. The adolescent brain. Dev Rev. 2008;28:62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester JA, de Paula Barrenha G, DeMaria A, Finegan A. Different effects of stress on alcohol drinking behaviour in male and female mice selectively bred for high alcohol preference. Alcohol Alcohol. 2006;41:44–53. doi: 10.1093/alcalc/agh242. [DOI] [PubMed] [Google Scholar]
- Corpechot C, Young J, Calvel M, Wehrey C, Veltz JN, Touyer G, Mouren M, Prasad VV, Banner C, Sjovall J, et al. Neurosteroids: 3 alpha-hydroxy-5 alpha-pregnan-20-one and its precursors in the brain, plasma, and steroidogenic glands of male and female rats. Endocrinology. 1993;133:1003–9. doi: 10.1210/endo.133.3.8365352. [DOI] [PubMed] [Google Scholar]
- Dahl RE, Gunnar MR. Heightened stress responsiveness and emotional reactivity during pubertal maturation: implications for psychopathology. Dev Psychopathol. 2009;21:1–6. doi: 10.1017/S0954579409000017. [DOI] [PubMed] [Google Scholar]
- Dalla C, Antoniou K, Drossopoulou G, Xagoraris M, Kokras N, Sfikakis A, Papadopoulou-Daifoti Z. Chronic mild stress impact: are females more vulnerable? Neuroscience. 2005;135:703–14. doi: 10.1016/j.neuroscience.2005.06.068. [DOI] [PubMed] [Google Scholar]
- Dalla C, Antoniou K, Kokras N, Drossopoulou G, Papathanasiou G, Bekris S, Daskas S, Papadopoulou-Daifoti Z. Sex differences in the effects of two stress paradigms on dopaminergic neurotransmission. Physiol Behav. 2008;93:595–605. doi: 10.1016/j.physbeh.2007.10.020. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Goldstein RB, Chou SP, Ruan WJ, Grant BF. Age at first drink and the first incidence of adult-onset DSM-IV alcohol use disorders. Alcohol Clin Exp Res. 2008;32:2149–60. doi: 10.1111/j.1530-0277.2008.00806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol Clin Exp Res. 2005;29:1796–808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Social and non-social anxiety in adolescent and adult rats after repeated restraint. Physiology & behavior. 2009;97:484–94. doi: 10.1016/j.physbeh.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards AC, Heron J, Dick DM, Hickman M, Lewis G, Macleod J, Kendler KS. Adolescent alcohol use is positively associated with later depression in a population-based U.K. cohort. J Stud Alcohol Drugs. 2014;75:758–65. doi: 10.15288/jsad.2014.75.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiland L, Romeo RD. Stress and the developing adolescent brain. Neuroscience. 2013;249:162–71. doi: 10.1016/j.neuroscience.2012.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Fudge JL. A developmental neurobiological model of motivated behavior: anatomy, connectivity and ontogeny of the triadic nodes. Neuroscience and biobehavioral reviews. 2009;33:367–82. doi: 10.1016/j.neubiorev.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eser D, Baghai TC, Schule C, Nothdurfter C, Rupprecht R. Neuroactive steroids as endogenous modulators of anxiety. Current pharmaceutical design. 2008;14:3525–33. doi: 10.2174/138161208786848838. [DOI] [PubMed] [Google Scholar]
- Faden VB. Trends in initiation of alcohol use in the United States 1975 to 2003. Alcoholism, clinical and experimental research. 2006;30:1011–22. doi: 10.1111/j.1530-0277.2006.00115.x. [DOI] [PubMed] [Google Scholar]
- Falk DE, Yi HY, Hilton ME. Age of onset and temporal sequencing of lifetime DSM-IV alcohol use disorders relative to comorbid mood and anxiety disorders. Drug Alcohol Depend. 2008;94:234–45. doi: 10.1016/j.drugalcdep.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidalgo TM, da Silveira ED, da Silveira DX. Psychiatric comorbidity related to alcohol use among adolescents. The American journal of drug and alcohol abuse. 2008;34:83–9. doi: 10.1080/00952990701764664. [DOI] [PubMed] [Google Scholar]
- Franceschelli A, Herchick S, Thelen C, Papadopoulou-Daifoti Z, Pitychoutis PM. Sex differences in the chronic mild stress model of depression. Behav Pharmacol. 2014;25:372–83. doi: 10.1097/FBP.0000000000000062. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Hippocampal 3alpha,5alpha-THP may alter depressive behavior of pregnant and lactating rats. Pharmacol Biochem Behav. 2004;78:531–40. doi: 10.1016/j.pbb.2004.03.024. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. Journal of substance abuse. 1997;9:103–10. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou PS, Kay W, Pickering R. The Alcohol Use Disorder and Associated Disabilities Interview Schedule-IV (AUDADIS-IV): reliability of alcohol consumption, tobacco use, family history of depression and psychiatric diagnostic modules in a general population sample. Drug and alcohol dependence. 2003;71:7–16. doi: 10.1016/s0376-8716(03)00070-x. [DOI] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Harford TC. Age at onset of alcohol use and DSM-IV alcohol abuse and dependence: a 12-year follow-up. Journal of substance abuse. 2001;13:493–504. doi: 10.1016/s0899-3289(01)00096-7. [DOI] [PubMed] [Google Scholar]
- Hargreaves GA, Wang EY, Lawrence AJ, McGregor IS. Beer promotes high levels of alcohol intake in adolescent and adult alcohol-preferring rats. Alcohol. 2011;45:485–98. doi: 10.1016/j.alcohol.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Henningsen K, Andreasen JT, Bouzinova EV, Jayatissa MN, Jensen MS, Redrobe JP, Wiborg O. Cognitive deficits in the rat chronic mild stress model for depression: relation to anhedonic-like responses. Behav Brain Res. 2009;198:136–41. doi: 10.1016/j.bbr.2008.10.039. [DOI] [PubMed] [Google Scholar]
- Heshmati M, Russo SJ. Anhedonia and the brain reward circuitry in depression. Curr Behav Neurosci Rep. 2015;2:146–153. doi: 10.1007/s40473-015-0044-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingson RW, Heeren T, Winter MR. Age at drinking onset and alcohol dependence: age at onset, duration, and severity. Archives of pediatrics & adolescent medicine. 2006;160:739–46. doi: 10.1001/archpedi.160.7.739. [DOI] [PubMed] [Google Scholar]
- Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicology and teratology. 1992;14:221–8. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- Ji D, Gilpin NW, Richardson HN, Rivier CL, Koob GF. Effects of naltrexone, duloxetine, and a corticotropin-releasing factor type 1 receptor antagonist on binge-like alcohol drinking in rats. Behavioural pharmacology. 2008;19:1–12. doi: 10.1097/FBP.0b013e3282f3cf70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national results on adolescent drug use: Overview of key findings, 2012. Ann Arbor: Institute for Social Research, The University of Michigan; 2013. [Google Scholar]
- Kamper EF, Chatzigeorgiou A, Tsimpoukidi O, Kamper M, Dalla C, Pitychoutis PM, Papadopoulou-Daifoti Z. Sex differences in oxidant/antioxidant balance under a chronic mild stress regime. Physiol Behav. 2009;98:215–22. doi: 10.1016/j.physbeh.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Crum RM, Warner LA, Nelson CB, Schulenberg J, Anthony JC. Lifetime co-occurrence of DSM-III-R alcohol abuse and dependence with other psychiatric disorders in the National Comorbidity Survey. Arch Gen Psychiatry. 1997;54:313–21. doi: 10.1001/archpsyc.1997.01830160031005. [DOI] [PubMed] [Google Scholar]
- Khisti RT, Chopde CT, Jain SP. Antidepressant-like effect of the neurosteroid 3alpha-hydroxy-5alpha-pregnan-20-one in mice forced swim test. Pharmacol Biochem Behav. 2000;67:137–43. doi: 10.1016/s0091-3057(00)00300-2. [DOI] [PubMed] [Google Scholar]
- Kushner MG, Abrams K, Borchardt C. The relationship between anxiety disorders and alcohol use disorders: a review of major perspectives and findings. Clin Psychol Rev. 2000;20:149–71. doi: 10.1016/s0272-7358(99)00027-6. [DOI] [PubMed] [Google Scholar]
- Lopez B, Turner RJ, Saavedra LM. Anxiety and risk for substance dependence among late adolescents/young adults. Journal of anxiety disorders. 2005;19:275–94. doi: 10.1016/j.janxdis.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Low NC, Lee SS, Johnson JG, Williams JB, Harris ES. The association between anxiety and alcohol versus cannabis abuse disorders among adolescents in primary care settings. Family practice. 2008;25:321–7. doi: 10.1093/fampra/cmn049. [DOI] [PubMed] [Google Scholar]
- Marmorstein NR. Longitudinal associations between alcohol problems and depressive symptoms: early adolescence through early adulthood. Alcohol Clin Exp Res. 2009;33:49–59. doi: 10.1111/j.1530-0277.2008.00810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CS, Lynch KG, Pollock NK, Clark DB. Gender differences and similarities in the personality correlates of adolescent alcohol problems. Psychology of addictive behaviors: journal of the Society of Psychologists in Addictive Behaviors. 2000;14:121–33. doi: 10.1037//0893-164x.14.2.121. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Mathews IZ. HPA function in adolescence: role of sex hormones in its regulation and the enduring consequences of exposure to stressors. Pharmacology, biochemistry, and behavior. 2007;86:220–33. doi: 10.1016/j.pbb.2006.07.012. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Mathews IZ, Thomas C, Waters P. Investigations of HPA function and the enduring consequences of stressors in adolescence in animal models. Brain Cogn. 2010;72:73–85. doi: 10.1016/j.bandc.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Molina-Hernandez M, Tellez-Alcantara NP, Garcia JP, Lopez JI, Jaramillo MT. Antidepressant-like actions of intra-accumbens infusions of allopregnanolone in ovariectomized Wistar rats. Pharmacol Biochem Behav. 2005;80:401–9. doi: 10.1016/j.pbb.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Morales M, Varlinskaya EI, Spear LP. Low doses of the NMDA receptor antagonists, MK-801, PEAQX, and ifenprodil, induces social facilitation in adolescent male rats. Behav Brain Res. 2013;250:18–22. doi: 10.1016/j.bbr.2013.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau JL, Scherschlicht R, Jenck F, Martin JR. Chronic mild stress-induced anhedonia model of depression; sleep abnormalities and curative effects of electroshock treatment. Behav Pharmacol. 1995;6:682–687. [PubMed] [Google Scholar]
- Morris EP, Stewart SH, Ham LS. The relationship between social anxiety disorder and alcohol use disorders: a critical review. Clinical psychology review. 2005;25:734–60. doi: 10.1016/j.cpr.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Oldehinkel AJ, Bouma EM. Sensitivity to the depressogenic effect of stress and HPA-axis reactivity in adolescence: a review of gender differences. Neuroscience and biobehavioral reviews. 2011;35:1757–70. doi: 10.1016/j.neubiorev.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Palmer RH, Young SE, Hopfer CJ, Corley RP, Stallings MC, Crowley TJ, Hewitt JK. Developmental epidemiology of drug use and abuse in adolescence and young adulthood: Evidence of generalized risk. Drug and alcohol dependence. 2009;102:78–87. doi: 10.1016/j.drugalcdep.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Sakharkar AJ, Tang L, Zhang H. Potential role of adolescent alcohol exposure-induced amygdaloid histone modifications in anxiety and alcohol intake during adulthood. Neurobiol Dis. 2015;82:607–19. doi: 10.1016/j.nbd.2015.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Boix J, Felipo V, Guerri C. Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. J Neurochem. 2009;108:920–31. doi: 10.1111/j.1471-4159.2008.05835.x. [DOI] [PubMed] [Google Scholar]
- Patrick ME, Schulenberg JE, Martz ME, Maggs JL, O’Malley PM, Johnston LD. Extreme binge drinking among 12th-grade students in the United States: prevalence and predictors. JAMA Pediatr. 2013;167:1019–25. doi: 10.1001/jamapediatrics.2013.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautassi RM, Myers M, Spear LP, Molina JC, Spear NE. Adolescent but not adult rats exhibit ethanol-mediated appetitive second-order conditioning. Alcohol Clin Exp Res. 2008;32:2016–27. doi: 10.1111/j.1530-0277.2008.00789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AC, Silbereisen RK, Sorensen S. Adolescent development: A global perspective. In: Hurrelmann K, Hamilton SF, editors. Social Problems and Social Contexts in Adolescence. Aldine de Gruyter; New York: 1996. pp. 3–37. [Google Scholar]
- Poulin JF, Laforest S, Drolet G. Enkephalin downregulation in the nucleus accumbens underlies chronic stress-induced anhedonia. Stress. 2014;17:88–96. doi: 10.3109/10253890.2013.850669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitychoutis PM, Dalla C, Sideris AC, Tsonis PA, Papadopoulou-Daifoti Z. 5-HT(1A), 5-HT(2A), and 5-HT(2C) receptor mRNA modulation by antidepressant treatment in the chronic mild stress model of depression: sex differences exposed. Neuroscience. 2012;210:152–67. doi: 10.1016/j.neuroscience.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Przybycien-Szymanska MM, Mott NN, Paul CR, Gillespie RA, Pak TR. Binge-pattern alcohol exposure during puberty induces long-term changes in HPA axis reactivity. PLoS One. 2011;6:e18350. doi: 10.1371/journal.pone.0018350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez RL, Spear LP. Ontogeny of ethanol-induced motor impairment following acute ethanol: assessment via the negative geotaxis reflex in adolescent and adult rats. Pharmacol Biochem Behav. 2010;95:242–8. doi: 10.1016/j.pbb.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo RD. Pubertal maturation and programming of hypothalamic-pituitary-adrenal reactivity. Front Neuroendocrinol. 2010;31:232–40. doi: 10.1016/j.yfrne.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Bellani R, Karatsoreos IN, Chhua N, Vernov M, Conrad CD, McEwen BS. Stress history and pubertal development interact to shape hypothalamic-pituitary-adrenal axis plasticity. Endocrinology. 2006;147:1664–74. doi: 10.1210/en.2005-1432. [DOI] [PubMed] [Google Scholar]
- Rutledge PC, Sher KJ. Heavy drinking from the freshman year into early young adulthood: the roles of stress, tension-reduction drinking motives, gender and personality. Journal of studies on alcohol. 2001;62:457–66. doi: 10.15288/jsa.2001.62.457. [DOI] [PubMed] [Google Scholar]
- Siciliano D, Smith RF. Periadolescent alcohol alters adult behavioral characteristics in the rat. Physiol Behav. 2001;74:637–43. doi: 10.1016/s0031-9384(01)00623-0. [DOI] [PubMed] [Google Scholar]
- Schmidt NB, Buckner JD, Keough ME. Anxiety sensitivity as a prospective predictor of alcohol use disorders. Behavior modification. 2007;31:202–19. doi: 10.1177/0145445506297019. [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Francis R, MacDonald A, Keistler C, O’Neill L, Kuhn CM. Effect of sex on ethanol consumption and conditioned taste aversion in adolescent and adult rats. Psychopharmacology (Berl) 2014;231:1831–9. doi: 10.1007/s00213-013-3319-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher L. The role of the hypothalamic-pituitary-adrenal axis dysfunction in the pathophysiology of alcohol misuse and suicidal behavior in adolescents. Int J Adolesc Med Health. 2007;19:3–9. doi: 10.1515/ijamh.2007.19.1.3. [DOI] [PubMed] [Google Scholar]
- Silveri MM. Adolescent brain development and underage drinking in the United States: identifying risks of alcohol use in college populations. Harvard review of psychiatry. 2012;20:189–200. doi: 10.3109/10673229.2012.714642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcoholism, clinical and experimental research. 1998;22:670–6. doi: 10.1111/j.1530-0277.1998.tb04310.x. [DOI] [PubMed] [Google Scholar]
- Siviy SM, Line BS, Darcy EA. Effects of MK-801 on rough-and-tumble play in juvenile rats. Physiol Behav. 1995;57:843–7. doi: 10.1016/0031-9384(94)00361-8. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and biobehavioral reviews. 2000;24:417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. Adolescent neurobehavioral characteristics, alcohol sensitivities, and intake: Setting the stage for alcohol use disorders? Child development perspectives. 2011;5:231–238. doi: 10.1111/j.1750-8606.2011.00182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. Adolescents and alcohol: acute sensitivities, enhanced intake, and later consequences. Neurotoxicol Teratol. 2014;41:51–9. doi: 10.1016/j.ntt.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. Adolescent alcohol exposure: Are there separable vulnerable periods within adolescence? Physiol Behav. 2015;148:122–30. doi: 10.1016/j.physbeh.2015.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. Alcohol consumption in adolescence: A translational perspective. Current Addiction Reports under revision. [Google Scholar]
- Spear LP, Swartzwelder HS. Adolescent alcohol exposure and persistence of adolescent-typical phenotypes into adulthood: a mini-review. Neurosci Biobehav Rev. 2014;45:1–8. doi: 10.1016/j.neubiorev.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP, Varlinskaya EI. Adolescence. Alcohol sensitivity, tolerance, and intake. Recent Dev Alcohol. 2005;17:143–59. [PubMed] [Google Scholar]
- Spear LP, Varlinskaya EI. Sensitivity to ethanol and other hedonic stimuli in an animal model of adolescence: implications for prevention science? Dev Psychobiol. 2010;52:236–43. doi: 10.1002/dev.20457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2007 National Survey on Drug Use and Health: National Findings. Rockville, MD: 2008. (Office of Applied Studies NSDUH Series H-34, DHHS Publication No SMA 08-4343). [Google Scholar]
- Tolliver GA, Samson HH. The influence of early postweaning ethanol exposure on oral self-administration behavior in the rat. Pharmacol Biochem Behav. 1991;38:575–80. doi: 10.1016/0091-3057(91)90016-u. [DOI] [PubMed] [Google Scholar]
- Torres JM, Ruiz E, Ortega E. Effects of CRH and ACTH administration on plasma and brain neurosteroid levels. Neurochemical research. 2001;26:555–8. doi: 10.1023/a:1010925331768. [DOI] [PubMed] [Google Scholar]
- Trezza V, Baarendse PJ, Vanderschuren LJ. Prosocial effects of nicotine and ethanol in adolescent rats through partially dissociable neurobehavioral mechanisms. Neuropsychopharmacology. 2009;34:2560–73. doi: 10.1038/npp.2009.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, Baarendse PJ, Vanderschuren LJ. The pleasures of play: pharmacological insights into social reward mechanisms. Trends Pharmacol Sci. 2010;31:463–9. doi: 10.1016/j.tips.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, Vanderschuren LJ. Bidirectional cannabinoid modulation of social behavior in adolescent rats. Psychopharmacology (Berl) 2008a;197:217–27. doi: 10.1007/s00213-007-1025-3. [DOI] [PubMed] [Google Scholar]
- Trezza V, Vanderschuren LJ. Cannabinoid and opioid modulation of social play behavior in adolescent rats: differential behavioral mechanisms. Eur Neuropsychopharmacol. 2008b;18:519–30. doi: 10.1016/j.euroneuro.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ufer M, Dadmarz M, Vogel WH. Voluntary consumption of amphetamine, cocaine, ethanol and morphine by rats as influenced by a preceding period of forced drug intake and clozapine. Pharmacology. 1999;58:285–91. doi: 10.1159/000028293. [DOI] [PubMed] [Google Scholar]
- Uhart M, Wand GS. Stress, alcohol and drug interaction: an update of human research. Addict Biol. 2009;14:43–64. doi: 10.1111/j.1369-1600.2008.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Doremus-Fitzwater TL, Spear LP. Repeated restraint stress alters sensitivity to the social consequences of ethanol in adolescent and adult rats. Pharmacol Biochem Behav. 2010;96:228–35. doi: 10.1016/j.pbb.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Acute effects of ethanol on social behavior of adolescent and adult rats: role of familiarity of the test situation. Alcoholism, clinical and experimental research. 2002;26:1502–11. doi: 10.1097/01.ALC.0000034033.95701.E3. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Differences in the social consequences of ethanol emerge during the course of adolescence in rats: social facilitation, social inhibition, and anxiolysis. Developmental psychobiology. 2006;48:146–61. doi: 10.1002/dev.20124. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Chronic tolerance to the social consequences of ethanol in adolescent and adult Sprague-Dawley rats. Neurotoxicology and teratology. 2007;29:23–30. doi: 10.1016/j.ntt.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Social interactions in adolescent and adult Sprague-Dawley rats: impact of social deprivation and test context familiarity. Behavioural brain research. 2008;188:398–405. doi: 10.1016/j.bbr.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Ethanol-induced social facilitation in adolescent rats: role of endogenous activity at mu opioid receptors. Alcohol Clin Exp Res. 2009;33:991–1000. doi: 10.1111/j.1530-0277.2009.00920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Social consequences of ethanol: Impact of age, stress, and prior history of ethanol exposure. Physiol Behav. 2015;148:145–50. doi: 10.1016/j.physbeh.2014.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Doremus-Fitzwater TL, Spear LP. Repeated restraint stress alters sensitivity to the social consequences of ethanol in adolescent and adult rats. Pharmacology, biochemistry, and behavior. 2010;96:228–35. doi: 10.1016/j.pbb.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Truxell EM, Spear LP. Repeated restraint stress alters sensitivity to the social consequences of ethanol differentially in early and late adolescent rats. Pharmacol Biochem Behav. 2013;113:38–45. doi: 10.1016/j.pbb.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Truxell E, Spear LP. Chronic intermittent ethanol exposure during adolescence: effects on social behavior and ethanol sensitivity in adulthood. Alcohol. 2014;48:433–44. doi: 10.1016/j.alcohol.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Truxell EM, Spear LP. Ethanol intake under social circumstances or alone in sprague-dawley rats: impact of age, sex, social activity, and social anxiety-like behavior. Alcohol Clin Exp Res. 2015a;39:117–25. doi: 10.1111/acer.12604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Truxell EM, Spear LP. Sex differences in sensitivity to the social consequences of acute ethanol and social drinking during adolescence. Behav Brain Res. 2015b;282:6–13. doi: 10.1016/j.bbr.2014.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter CS, Doremus-Fitzwater TL, Spear LP. Time course of elevated ethanol intake in adolescent relative to adult rats under continuous, voluntary-access conditions. Alcohol Clin Exp Res. 2007;31:1159–68. doi: 10.1111/j.1530-0277.2007.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter-O’Hagen CS, Spear LP. Hormonal and physical markers of puberty and their relationship to adolescent-typical novelty-directed behavior. Developmental psychobiology. 2012;54:523–35. doi: 10.1002/dev.20610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter-O’Hagen C, Varlinskaya E, Spear L. Sex differences in ethanol intake and sensitivity to aversive effects during adolescence and adulthood. Alcohol and alcoholism. 2009;44:547–54. doi: 10.1093/alcalc/agp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells JE, Horwood LJ, Fergusson DM. Drinking patterns in mid-adolescence and psychosocial outcomes in late adolescence and early adulthood. Addiction. 2004;99:1529–41. doi: 10.1111/j.1360-0443.2004.00918.x. [DOI] [PubMed] [Google Scholar]
- Willey AR, Varlinskaya EI, Spear LP. Social interactions and 50 kHz ultrasonic vocalizations in adolescent and adult rats. Behav Brain Res. 2009;202:122–9. doi: 10.1016/j.bbr.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology (Berl) 1997;134:319–29. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- Zehe JM, Colder CR, Read JP, Wieczorek WF, Lengua LJ. Social and generalized anxiety symptoms and alcohol and cigarette use in early adolescence: the moderating role of perceived peer norms. Addict Behav. 2013;38:1931–9. doi: 10.1016/j.addbeh.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP. Multiparous species present problems (and possibilities) to developmentalists. Developmental psychobiology. 1997;30:141–50. doi: 10.1002/(sici)1098-2302(199703)30:2<141::aid-dev5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]


