Abstract
Rotavirus A (RVA) is a dominant causative agent of acute gastroenteritis in children worldwide. G2P[4] is one of the most common genotypes among human rotavirus (HRV) strains, and has been persistently prevalent in South Asia including Bangladesh. In the present study, whole genome sequences of a total of 16 G2P[4] HRV strains (8 strains each in 2010 and 2013) detected in Mymensingh, north-central Bangladesh were determined. These strains had typical DS-1-like genotype constellation. Most of gene segments from DS-1 genogroup exhibited high level sequence identities to each other (>98%), while slight diversity was observed for VP1, VP3, and NSP4 genes. By phylogenetic analysis, individual RNA segments were classified into one (V) or two-three lineages (V–VI or V–VII). In terms of lineages (sublineages) of 11 gene segments, the 16 Bangladeshi strains could be further classified into four clades (A-D) containing 8 lineage constellations, revealing the presence of three clades (A-C) with three lineage constellations in 2010, and a single clade (D) with four constellations in 2013. Therefore, co-existence of multiple G2P[4] HRV strains with different lineage constellations, and change in clades for the study period were demonstrated. Although amino acids in the antigenic regions on VP7 and VP4 were mostly identical to those of global G2P[4] strains after 2000, VP4 of clade D RVAs in 2013 had alanine and proline at positions 88 and 114, respectively, which are novel substitutions compared with recent global G2P[4] strains. Replacement of lineage constellations associated with unique amino acid changes in the antigenic region in VP4 suggested continuous genetic evolutionary state for emerging new G2P[4] rotavirus strains in Bangladesh.
Keywords: Evolution, Genetics, Microbiology
1. Introduction
Rotavirus A (Group A rotavirus, RVA) is the leading etiological agent of severe gastroenteritis in infants and young children worldwide, and is estimated to cause 197,000 deaths in children <5 years of age (Lanata et al., 2013). As enteric pathogens, RVAs circulate in mammals and birds. Rotavirus is a genus of the family Reoviridae, and its genome is composed of 11 segments of double-stranded RNA enclosed in a triple-layered capsid. RNA segments of RVA encode six structural proteins (VP1-VP4, VP6 and VP7) and six nonstructural proteins (NSP1-NSP6) (Estes and Greenberg, 2013). Due to the segmented nature of the rotavirus genome, reassortment is considered to occur occasionally when co-infection with more than one rotavirus genotype occurs in hosts (Greenberg et al., 1981; Midthun et al., 1987; Urasawa et al., 1986). This reassortment has been revealed by whole genomic analysis (Ghosh and Kobayashi, 2011a).
The outermost layer of the rotavirus particle consists of two structural proteins VP7 and VP4, on which neutralization antigens are present and define independent serotypes (G and P serotypes, respectively). Based on the VP7 and VP4 genes, RVA has been genetically classified into G type and P type, respectively (Estes and Greenberg, 2013). A total of 27 G types and 37 P types have been described to date for human and animal rotaviruses (Matthijnssens et al., 2011; Trojnar et al., 2013). Genotypes G1P[8], G2P[4], G3P[8], G4P[8], G9P[8], G12P[8] and combinations thereof, are frequently detected in human RVAs throughout the world (Santos and Hoshino, 2005; Dóró et al., 2014). A comprehensive genetic analysis of all 11 segments revealed that there are two major genotype constellations in human rotaviruses (HRVs). The Wa genogroup (Wa-like genotype constellation) includes strains with G1P[8], G3P[8], or G4P[8] genotypes, and the DS-1 genogroup (DS-1-like genotype constellation) is usually associated with G2P[4] HRV strains (Matthijnssens et al., 2008).
G2P[4], one of the most common HRV genotypes worldwide, has been showing relatively high detection rates (16–36%) in South Asia recently (Dóró et al., 2014; Miles et al., 2012; Mullick et al., 2014). In Bangladesh, G2 has occasionally been the most common genotype among circulating HRVs for the past 20 years, while G9 and G12 emerged as predominant strains in the last decade (Afrad et al., 2013; Afrad et al., 2014; Ahmed et al., 2012; Dey et al., 2009; Miles et al., 2012; Paul et al., 2008). The reason for the occasional dominance of G2P[4] HRV in Bangladesh has not yet been fully understood, although the occurrence of multiple lineages of VP7 gene is suggested to be one of the possible reasons (Afrad et al., 2014).
G2P[4] genotype has been known to increase or persist in dominance after introduction of monovalent rotavirus vaccine in Brazil, Argentina, Australia, Belgium, and Korea (Gurgel et al., 2014; Kim et al., 2014; Kirkwood et al., 2009; Kirkwood et al., 2011; Mandile et al., 2014; Matthijnssens et al., 2014). Therefore, it is important to understand diversity and genetic evolution of G2P[4] HRVs in nature and their association with efficacy of vaccines. Whole genomic analysis of HRVs revealed presence of three distinct clades among G2P[4] in 2010–2011 winter season in the USA (Dennis et al., 2014). Furthermore, long-term investigations of whole genome of G2P[4] HRVs in Italy (Giammanco et al., 2014) and Japan (Doan et al., 2015) indicated occurrence of major genomic change in the global G2P[4] RVAs in the early 2000s.
In Bangladesh, genetic diversity and evolution of G2P[4] RVAs was analyzed in terms of VP7 gene from a large number of G2P[4] strains (Afrad et al., 2014). In this study, Afrad and coworkers revealed that multiple lineages of G1 and G2 HRVs had been co-circulating over the years. Whole genomes of HRV G1, G2 and G12 strains in Bangladesh were previously analyzed (Ghosh et al., 2011b; Rahman et al., 2007; Rahman et al., 2010), with only two G2P[4] HRV strains available for study (Ghosh et al., 2011b). Thus, the genomic diversity of G2P[4] HRVs in Bangladesh have not yet been well characterized using whole genome sequencing. The purpose of the present study was to elucidate evolutionary state of all the gene segments of G2P[4] HRVs, i.e., to understand genetic diversity of individual genome segments and correlation of evolution among gene segments. In the present study, we analyzed whole genome of 16 G2P[4] HRV strains in 2010 and 2013 to obtain clues to understand their persistence in Bangladesh.
2. Material and methods
2.1. Virus strains
A total of 17 G2P[4] HRV strains isolated from diarrheal stool samples collected from patients aged 3 months to 24 years in Mymensingh, Bangladesh were studied. Nine and eight HRV strains were obtained in Jan.–Feb. 2010 and Aug.–Dec. 2013, respectively (Table 1). In 2010, G2 was the most prevalent (41%), followed by G1 (25%), and G9 (8%), in Mymensingh (unpublished data). Genotyping results of HRV in 2013 have not yet been available. All the strains were confirmed to have G2P[4] genotypes by semi-nested RT-PCR as described previously (Iturriza-Gómara et al., 2004; Nagashima S et al., 2010). For the experiments, utmost attention was paid to avoid contamination especially in handling stool specimens, for example, using only one sample per day for RNA extraction and RT-PCR.
Table 1.
Date, age, and sex of the patients infected with RVA strains.
| RVA strain | Date of collection (year/month) | Age | Sex |
|---|---|---|---|
| RVA/Human-wt/BGN/J331/2010/G2P[4]* | 2010.2 | 1Y | M |
| RVA/Human-wt/BGN/J306/2010/G2P[4] | 2010.2 | 24Y | M |
| RVA/Human-wt/BGN/J303/2010/G2P[4] | 2010.2 | 12Y | M |
| RVA/Human-wt/BGN/J300/2010/G2P[4] | 2010.2 | 6Y | M |
| RVA/Human-wt/BGN/J266/2010/G2P[4] | 2010.1 | 20Y | M |
| RVA/Human-wt/BGN/J265/2010/G2P[4] | 2010.1 | 6M | M |
| RVA/Human-wt/BGN/J263/2010/G2P[4] | 2010.1 | 20Y | M |
| RVA/Human-wt/BGN/J253/2010/G2P[4] | 2010.1 | 5Y | M |
| RVA/Human-wt/BGN/J251/2010/G2P[4] | 2010.1 | 22Y | M |
| RVA/Human-wt/BGN/M334/2013/G2P[4] | 2013.9 | 8M | F |
| RVA/Human-wt/BGN/M315/2013/G2P[4] | 2013.8 | 4M | M |
| RVA/Human-wt/BGN/M313/2013/G2P[4] | 2013.8 | 10M | M |
| RVA/Human-wt/BGN/M312/2013/G2P[4] | 2013.8 | 2Y | M |
| RVA/Human-wt/BGN/M310/2013/G2P[4] | 2013.8 | 3Y | M |
| RVA/Human-wt/BGN/M292/2013/G2P[4] | 2013.12 | 3M | M |
| RVA/Human-wt/BGN/M289/2013/G2P[4] | 2013.11 | 5M | M |
| RVA/Human-wt/BGN/M282/2013/G2P[4] | 2013.11 | 6M | F |
*This strain had Wa-like genotypes in VP3 gene (M1) and NSP5/6 gene (H1) in the DS-1-like genotype constellation. Because the possibility of mixed infection could not be excluded, this strain was not included in the phylogenetic analysis.
2.2. Nucleotide sequencing, genotyping and sequence analyses
Viral RNA was extracted from stool samples using the QIAamp Viral RNA Mini Kit (Qiagen Science, MD, USA). RT-PCRs were performed using Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA) and Prime Star GXL DNA polymerase (TaKaRa, Japan). Primers used for amplification of viral genome segments have been described previously (Ghosh et al., 2010a; Ghosh et al., 2010b; Ghosh et al., 2011b). For the RT-PCR of VP1-4, NSP1, and NSP4 genes, additional primers were designed in the present study as shown in Table 2. Nucleotide sequences were determined by the Sanger method using the BigDye Terminator v3.1 Cycle Sequencing kit (Applied Biosystems, Foster City, CA) on an automated DNA sequencer (ABI PRISM 3100). The Basic Local Alignment Search Tool (BLAST) (http://blast.ncbi.nlm.nih.gov/Blast.cgi) was used to search for the most similar rotavirus gene sequence and assign its genotype based on cut-off values indicated by Rotavirus Classification Working Group (Matthijnssens et al., 2011). Phylogenetic trees of individual gene segments were constructed by Maximum Likelihood method using MEGA6 software (Tamura et al., 2013), with sequences selected from GenBank database. Phylogenetic trees were statistically supported by bootstrapping with 1000 replicates, and phylogenetic distances were measured by the Kimura two-parameter model (Kimura, 1980). Multiple alignments of sequences were performed using CLUSTAL W ver. 2.1 program available on website of DDBJ (http://clustalw.ddbj.nig.ac.jp/). Sequence identity of a pair of gene sequences was determined by using LALIGN program on web server (http://www.ch.embnet.org/software/LALIGN_form.html).
Table 2.
Primers designed in the present study.
| Gene segment | Primer | Sequence(5'–3') | Nucleotide Position* |
Size |
|---|---|---|---|---|
| VP1 | 390R | CAT CAA TGA GTC AGT GTA TTC | 408–388 | 21 mer |
| VP1 | 2640R | GGT TTT ATG TCT TTA AGT ATG TCG | 2666–2643 | 24 mer |
| VP2 | 480F | GGG GAC TAT GAT GTG AGA GAG | 485–505 | 21 mer |
| VP2 | 306F | CCA ACA TTC GAA CCT AAA GAG ACG | 299–322 | 24 mer |
| VP2 | 1014F | GGC GAG ATC GGT AGT ACC AG | 997–1016 | 20 mer |
| VP3 | 2195R | GTA CCA CAT CTC ACA TTT GGC G | 2195–2216 | 22 mer |
| VP3 | 1860R | CAC ATG TCC AGA CAC TGA ATT CTC | 1888–1865 | 24 mer |
| VP3 | 2427R | TCG TGA TTG TCC AAA CGT GAT G | 2425–2404 | 22 mer |
| VP3 | 1725R | CCC ATA TGA TTT GCA TAT TGA TC | 1752–1730 | 23 mer |
| VP3 | 2124F | ATA TAG TAT AAC TTA TGC TGA CG | 2113–2135 | 23 mer |
| VP4 | 2091F | GGA TAC ACT TAA TGA GAT CCC | 2091–2111 | 21 mer |
| VP4 | 1215F | CTA TTA TGA ATG GCG GTGCTG | 1190–1210 | 21 mer |
| NSP1 | 200R | ATG TTG ACA ACA ATC TAA GC | 175–156 | 20 mer |
| NSP1 | 600F | ATG TAT TAC TGC TAG ATA GC | 576–595 | 20 mer |
| NSP4 | 550F | AGA GGT TGA GCT GCC GTC GTC | 569–589 | 21 mer |
| NSP4 | 500R | TAG CGT TTT CAC GTT CTT TTG | 509–489 | 21 mer |
*These positions correspond to individual gene segments of G2P[4] HRV.
2.3. Nucleotide sequence accession numbers
The GenBank accession numbers for the nucleotide sequences determined in the present study were listed in Table 3.
Table 3.
GeneBank accesseion numbers for the 11gene segments of 16 G2P[4] RVA strains in Mymensingh, Bangladesh.
3. Results
The genotype constellation of 16 strains in this study was G2-P[4]-I2-R2-C2-M2-A2-N2-T2-E2-H2, showing typical DS-1-like genotype constellation. One strain J331 detected in 2010 had the genotype constellation G2-P[4]-I2-R2-C2-M1-A2-N2-T2-E2-H1, which contained genotypes of Wa genogroup (M1 and H1). Because the possibility of mixed infection with Wa genogroup HRV could not be excluded, strain J331 was not included in the phylogenetic analysis with other G2P[4] strains.
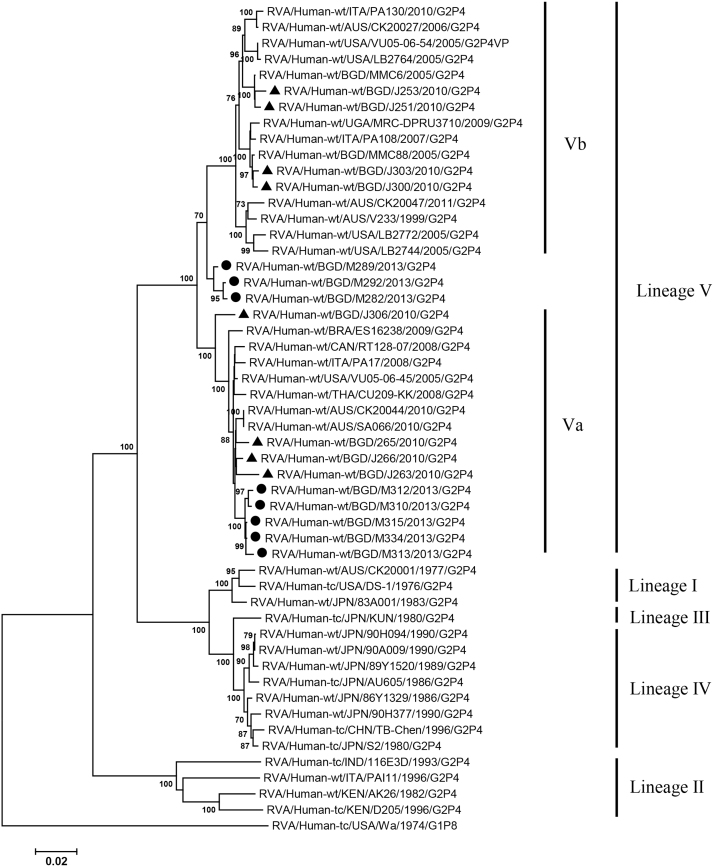
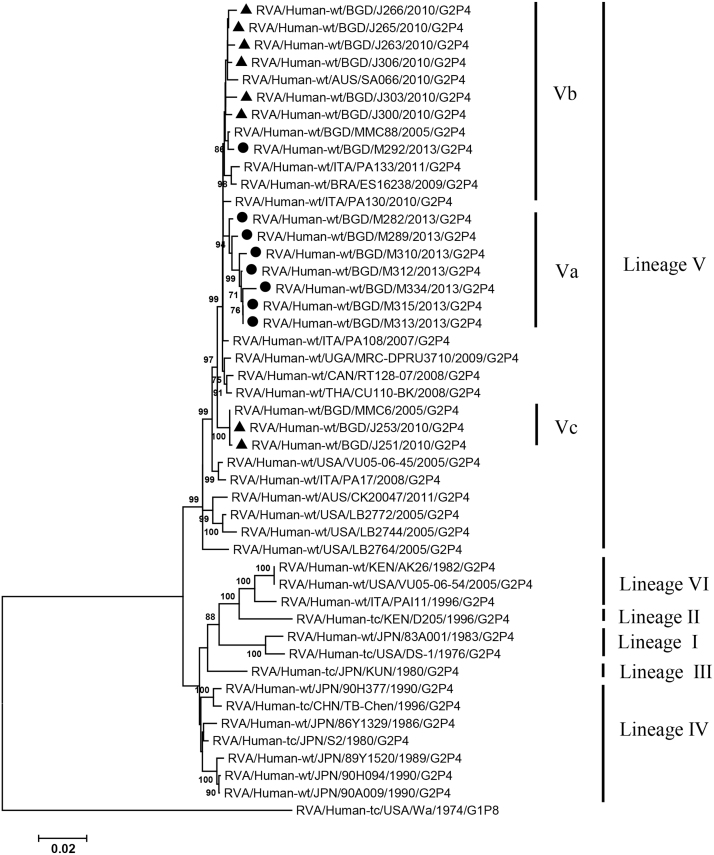
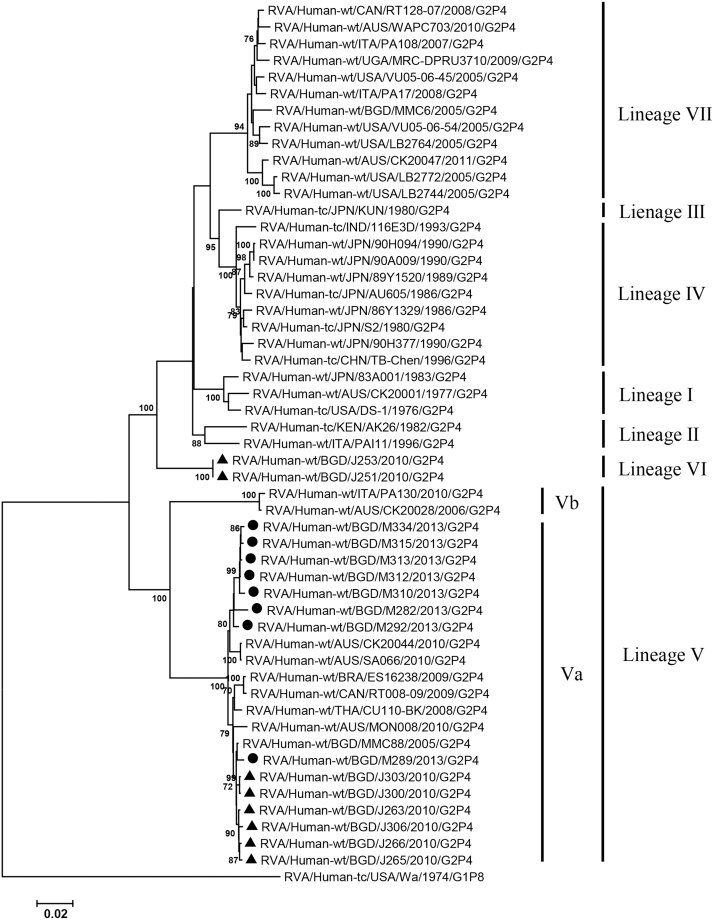
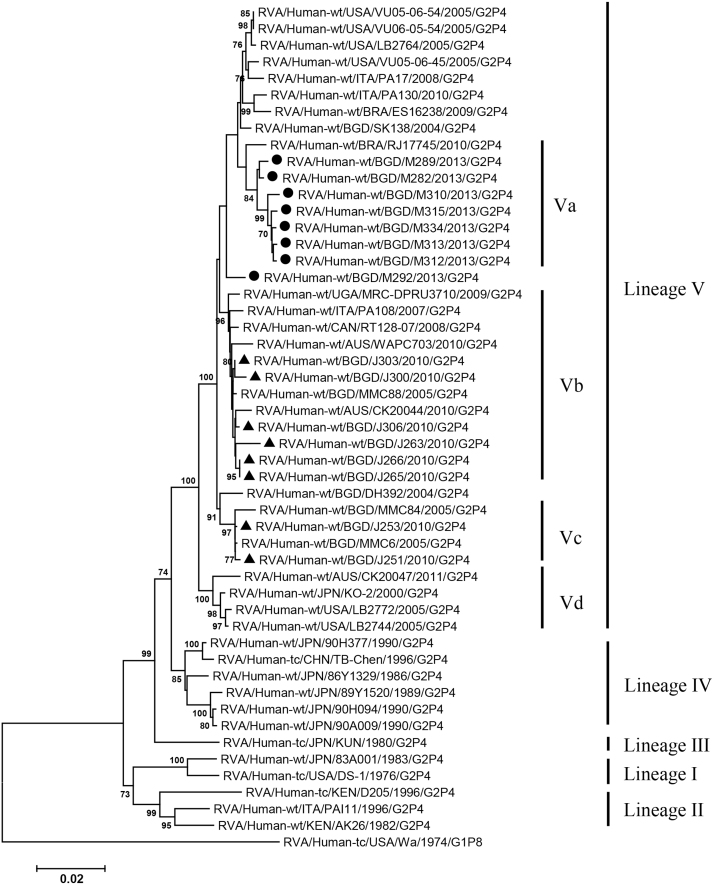
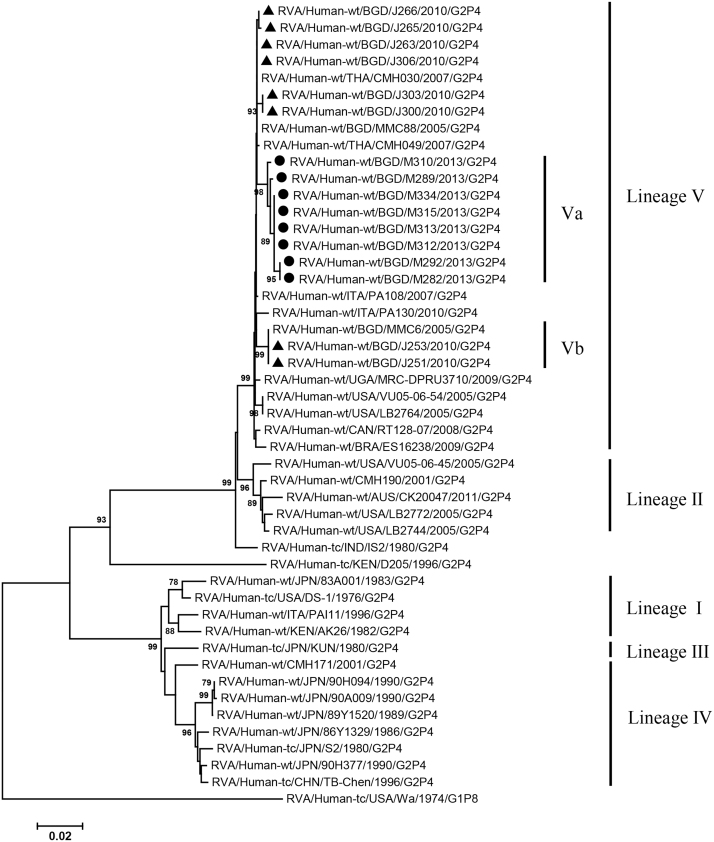
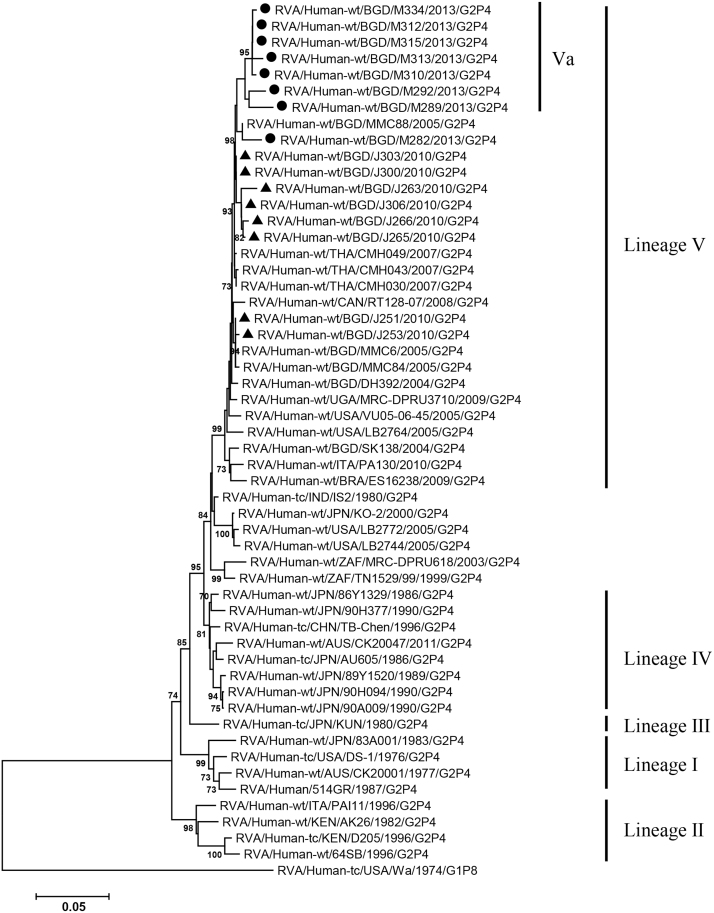
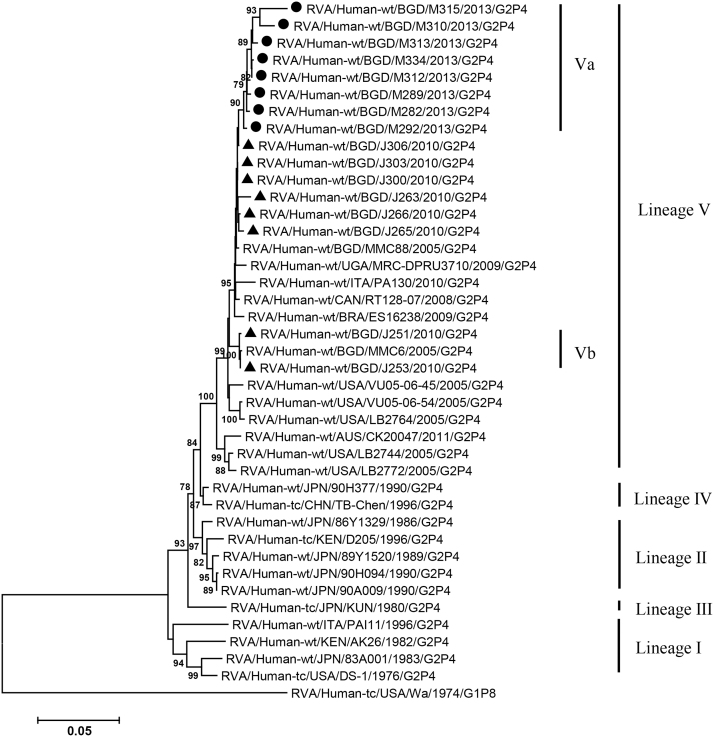
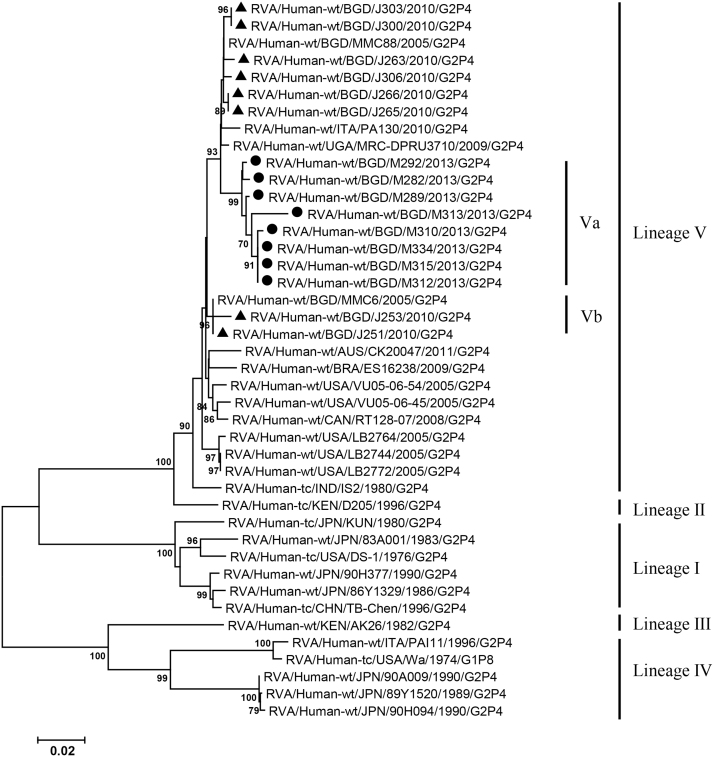
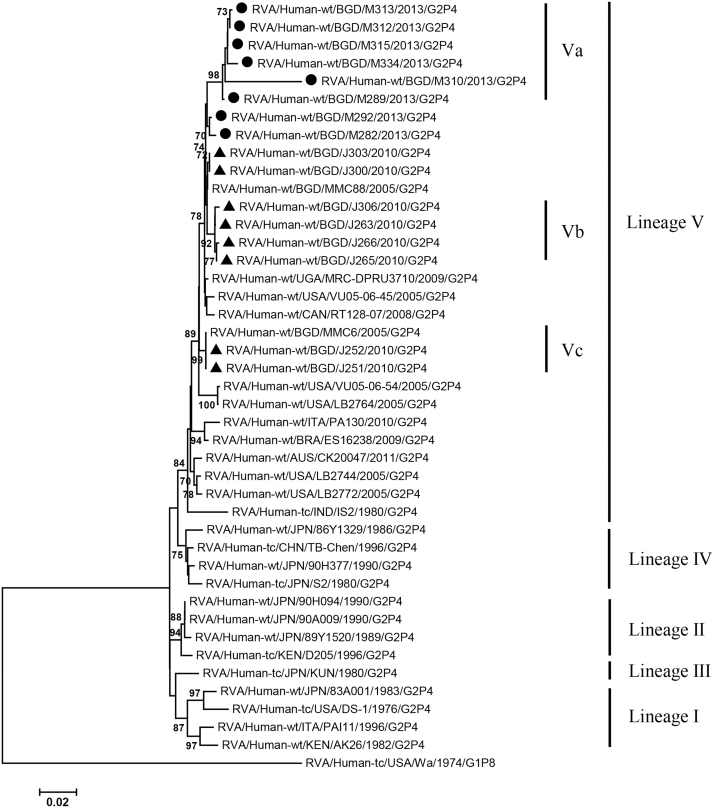
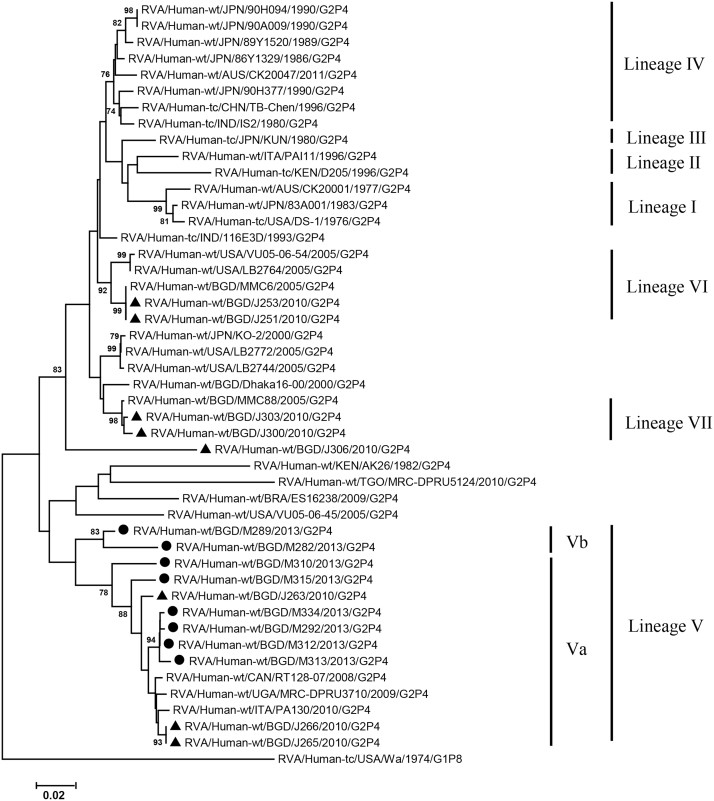
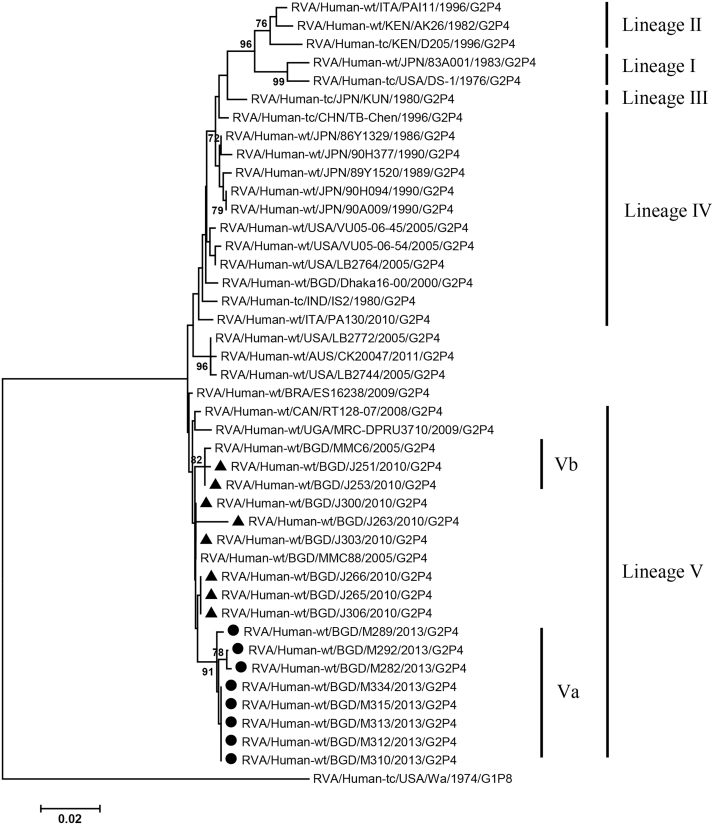
VP2, VP4, VP6, VP7, NSP1-3 and NSP5 genes from the 16 G2P[4] strains exhibited high level sequence conservation with >98% sequence identity to each other. In contrast, slight sequence diversity was observed for VP1, VP3, and NSP4 genes among the 16 strains (94.4%, 90.6%, and 94.3% sequence identity, respectively). Phylogenetic analysis together with representative G2P[4] RVAs from the world (Bányai et al., 2011; Chaimongkol et al., 2012; Doan et al., 2015; Ghosh et al., 2011b; Giammanco et al., 2014; Page and Steele, 2004) indicated that most of the Bangladeshi G2 HRV genome segments were classified into a single lineage (lineage V), while VP3 and NSP4 genes were assigned to two (V, VI) and three lineages (V–VII), respectively (Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7, Fig. 8, Fig. 9, Fig. 10, Fig. 11). We followed designation of lineages in each RNA segment as described by Doan et al. (2015).
Fig. 1.
Phylogenetic dendrograms based on full-length nucleotide sequences of genes encoding VP1. Bangladeshi RVA strains detected in 2010 and 2013 analyzed in the present study are marked with closed triangles and circles, respectively. Lineages and sublineages within a lineage are shown with vertical lines on the right. Lineages I–IV of individual genes were assigned by the scheme described by Doan et al. (2015), while other lineages and sublineages were designated in the present study. Scale bars are shown below. Bootstrap values are indicated at nodes of branches. Bootstrap values less than 70% are not shown.
Fig. 2.
Phylogenetic dendrograms based on full-length nucleotide sequences of genes encoding VP2. See legends of Fig. 1 for marks, lineage assignment, scale bars and bootstrap values.
Fig. 3.
Phylogenetic dendrograms based on full-length nucleotide sequences of genes encoding VP3. See legends of Fig. 1 for marks, lineage assignment, scale bars and bootstrap values.
Fig. 4.
Phylogenetic dendrograms based on full-length nucleotide sequences of genes encoding VP4. See legends of Fig. 1 for marks, lineage assignment, scale bars and bootstrap values.
Fig. 5.
Phylogenetic dendrograms based on full-length nucleotide sequences of genes encoding VP6. See legends of Fig. 1 for marks, lineage assignment, scale bars and bootstrap values.
Fig. 6.
Phylogenetic dendrograms based on full-length nucleotide sequences of genes encoding VP7. See legends of Fig. 1 for marks, lineage assignment, scale bars and bootstrap values.
Fig. 7.
Phylogenetic dendrograms based on full-length nucleotide sequences of genes encoding NSP1. See legends of Fig. 1 for marks, lineage assignment, scale bars and bootstrap values.
Fig. 8.
Phylogenetic dendrograms based on full-length nucleotide sequences of genes encoding NSP2. See legends of Fig. 1 for marks, lineage assignment, scale bars and bootstrap values.
Fig. 9.
Phylogenetic dendrograms based on full-length nucleotide sequences of genes encoding NSP3. See legends of Fig. 1 for marks, lineage assignment, scale bars and bootstrap values.
Fig. 10.
Phylogenetic dendrograms based on full-length nucleotide sequences of genes encoding NSP4. See legends of Fig. 1 for marks, lineage assignment, scale bars and bootstrap values.
Fig. 11.
Phylogenetic dendrograms based on full-length nucleotide sequences of genes encoding NSP5. See legends of Fig. 1 for marks, lineage assignment, scale bars and bootstrap values.
Within the lineage V, if the sequences of present Bangladeshi HRVs clustered in a branch supported with high bootstrap value, sublineage was designated with a subscript attached with lineage V (Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7, Fig. 8, Fig. 9, Fig. 10, Fig. 11). A subscript “a” was assigned to five strains in 2013 because they clustered together in all the gene segments, and subscript b and c were assigned arbitrarily. G2P[4] strains in 2013 had mostly gene segments belonging to sublineage Va, while several genes of three strains were not assigned to lineage Va. In contrast, G2P[4] strains in 2010 were more divergent than 2013 strains, because individual gene segments belonged to various sublineages or were not assigned to the sublineages. Based on combination of sublineages (lineages) in 11 gene segments, G2P[4] HRVs in 2010 and 2013 were classified into four lineage constellations each (Table 4). In terms of the similarity of lineage constellations (five or more identical sublineages of lineage V, and lineages VI and VII), the eight strains each in 2010 and 2013 were classified into three clades (A-C) and one clade (D), respectively.
Table 4.
Lineages (sublineages) of 11 genome segments detected in Mymensingh, Bangladesh.
| RVA strain | Lineage (sublineages)* of viral protein genes (genotype : G2-P[4]-I2-R2-C2-M2-A2-N2-T2-E2-H2) |
Lineage constellation# | Clade# | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VP7 | VP4 | VP6 | VP1 | VP2 | VP3 | NSP1 | NSP2 | NSP3 | NSP4 | NSP5/6 | |||
| RVA/Human-wt/BGN/MMC6/2005/G2P[4] | V | Vc | Vb | Vb | Vc | VII | Vb | Vb | Vc | VI | Vb | ||
| RVA/Human-wt/BGN/MMC88/2005/G2P[4] | V | Vb | V | Vb | Vb | Va | V | V | V | VII | V | ||
| RVA/Human-wt/BGN/J306/2010/G2P[4] | V | Vb | V | Va | Vb | Va | V | V | Vb | V | V | 2010–4 | A |
| RVA/Human-wt/BGN/J303/2010/G2P[4] | V | Vb | V | Vb | Vb | Va | V | V | V | VII | V | 2010–2 | B |
| RVA/Human-wt/BGN/J300/2010/G2P[4] | V | Vb | V | Vb | Vb | Va | V | V | V | VII | V | 2010–2 | B |
| RVA/Human-wt/BGN/J266/2010/G2P[4] | V | Vb | V | Va | Vb | Va | V | V | Vb | Va | V | 2010–1 | A |
| RVA/Human-wt/BGN/J265/2010/G2P[4] | V | Vb | V | Va | Vb | Va | V | V | Vb | Va | V | 2010–1 | A |
| RVA/Human-wt/BGN/J263/2010/G2P[4] | V | Vb | V | Va | Vb | Va | V | V | Vb | Va | V | 2010–1 | A |
| RVA/Human-wt/BGN/J253/2010/G2P[4] | V | Vc | Vb | Vb | Vc | VI | Vb | Vb | Vc | VI | Vb | 2010–3 | C |
| RVA/Human-wt/BGN/J251/2010/G2P[4] | V | Vc | Vb | Vb | Vc | VI | Vb | Vb | Vc | VI | Vb | 2010–3 | C |
| RVA/Human-wt/BGN/M334/2013/G2P[4] | Va | Va | Va | Va | Va | Va | Va | Va | Va | Va | Va | 2013–1 | D |
| RVA/Human-wt/BGN/M315/2013/G2P[4] | Va | Va | Va | Va | Va | Va | Va | Va | Va | Va | Va | 2013–1 | D |
| RVA/Human-wt/BGN/M313/2013/G2P[4] | Va | Va | Va | Va | Va | Va | Va | Va | Va | Va | Va | 2013–1 | D |
| RVA/Human-wt/BGN/M312/2013/G2P[4] | Va | Va | Va | Va | Va | Va | Va | Va | Va | Va | Va | 2013–1 | D |
| RVA/Human-wt/BGN/M310/2013/G2P[4] | Va | Va | Va | Va | Va | Va | Va | Va | Va | Va | Va | 2013–1 | D |
| RVA/Human-wt/BGN/M292/2013/G2P[4] | Va | V | Va | V | Vb | Va | Va | Va | V | Va | Va | 2013–2 | D |
| RVA/Human-wt/BGN/M289/2013/G2P[4] | Va | Va | Va | V | Va | Va | Va | Va | Va | Vb | Va | 2013–3 | D |
| RVA/Human-wt/BGN/M282/2013/G2P[4] | V | Va | Va | V | Va | Va | Va | Va | V | Vb | Va | 2013–4 | D |
Lineage (sublineage) is the same as that described in Fig. 1.
Lineage constellation was designated based on combination of lineages/sublineages of 11 genome segments, and clades A-D were defined as group of lineage constellations having five or more identical sublineages of lineage V (and lineage VI and VII in some strains).
All the genome segments of HRV strains analyzed in the present study clustered with those of G2P[4] HRV strains MMC6 and/or MMC88 detected in Bangladesh in 2005 (Ghosh et al., 2011b) and other contemporary G2P[4] strains from Americas, Asia, etc., within the lineage V, in the phylogenetic trees. However, clustering patterns with strains MMC6 and/or MMC88 were different depending on gene segments. Strains MMC6 and MMC88 had VP1, VP2, VP4, VP6, NSP1, NSP2, NSP5/6 genes classified into sublineages Vb or Vc. However, only VP3 gene of MMC88 was assigned to sublineage Va. VP7 gene of MMC6 and MMC88 did not cluster with the lineage Va. NSP4 genes of lineage VI (MMC6) and VII (MMC88) clustered with clade C and B strains, respectively.
Between sublineages Va and Vb, sequence identity of VP1 genes was approximately 94%, while >97.9 identity was found within the same sublineages. Lineage VI and VII NSP4 genes exhibited 94% identity to sublineage Va NSP4 genes. VP3 genes of sublineage Va showed 90.6% identity to those of lineage VI strains, in contrast to >99% identity among Va lineage.
Amino acid residues in VP7 defining neutralization domain of the G2P[4] strains in 2010 and 2013 were mostly identical to those of MMC88 strain in 2005, although one or two amino acid difference was found with strains J251 and J253 in 2010, and M313 and M289 in 2013 (Table 5). All the G2P[4] strains analyzed had 4–7 amino acids in the neutralization domain which are different from those of G2 component of pentavalent vaccine (RotaTeq) and G2 prototype strain DS-1, as described previously for recent G2P[4] rotaviruses (Afrad et al., 2014; Giammanco et al., 2014; Zeller et al., 2011). Although VP4 neutralization domains of G2P[4] HRVs in 2010 and 2013 were also similar to those of MMC88 strain, all the eight strains in 2013 had different amino acids at position 88 (A) and 114 (P) from those in the G2P[4] strains in 2005 and 2010 as well as DS-1 strain (Q and T, respectively) (Table 6a, Table 6b).
Table 5.
Alignment of the amino acid residues defining the neutralization domains of VP7 (7-1a, 7-1b, and 7–2) between RotaTeq™ G2 component and G2 strains DS-1, TB-Chen, MMC88, and 16 G2P[4] RVA strains in Bangladesh.
| RVA strain | 7-1a |
7-1b |
7-2 |
||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 87 | 91 | 94 | 96 | 97 | 98 | 99 | 100 | 104 | 123 | 125 | 129 | 130 | 291 | 201 | 211 | 212 | 213 | 238 | 242 | 143 | 145 | 146 | 147 | 148 | 190 | 217 | 221 | 264 | |||
| RVA/Vaccine/USA/RotaTeq SC2-9/1992/G2P[5] | A | N | S | D | E | W | E | N | Q | D | T | M | N | K | Q | D | V | S | N | S | R | D | N | T | S | D | I | S | G | ||
| RVA/Human-tc/USA/DS-1/1976/G2P[4] | A | N | S | D | E | W | E | N | Q | D | T | M | N | K | Q | D | V | D* | N | S | R | D | N | T | S | D | I | S | G | ||
| RVA/Human-tc/CHN/TB-Chen/1996/G2P[4] | A | N | S | D | E | W | E | N | Q | D | N | V | N | K | Q | D | V | N* | N | N* | R | D | N | T | S | D | I | S | G | ||
| RVA/Human-wt/BGN/MMC88/2005/G2P[4] | T* | N | S | N* | E | W | E | N | Q | D | T | M | D* | K | Q | D | V | D* | N | N* | R | D | N | T | S | D | I | S | G | ||
| RVA/Human-wt/BGN/J306/2010/G2P[4] | T* | N | S | N* | E | W | E | N | Q | D | T | M | D* | K | Q | D | V | D* | N | N* | R | D | N | T | S | D | I | S | G | ||
| RVA/Human-wt/BGN/J303/2010/G2P[4] | T* | N | S | N* | E | W | E | N | Q | D | T | M | D* | K | Q | D | V | D* | N | N* | R | D | N | T | S | D | I | S | G | ||
| RVA/Human-wt/BGN/J300/2010/G2P[4] | T* | N | S | N* | E | W | E | N | Q | D | T | M | D* | K | Q | D | V | D* | N | N* | R | D | N | T | S | D | I | S | G | ||
| RVA/Human-wt/BGN/J266/2010/G2P[4] | T* | N | S | N* | E | W | E | N | Q | D | T | M | D* | K | Q | D | V | D* | N | N* | R | D | N | T | S | D | I | S | G | ||
| RVA/Human-wt/BGN/J265/2010/G2P[4] | T* | N | S | N* | E | W | E | N | Q | D | T | M | D* | K | Q | D | V | D* | N | N* | R | D | N | T | S | D | I | S | G | ||
| RVA/Human-wt/BGN/J263/2010/G2P[4] | T* | N | S | N* | E | W | E | N | Q | D | T | M | D* | K | Q | D | V | D* | N | N* | R | D | N | T | S | D | I | S | G | ||
| RVA/Human-wt/BGN/J253/2010/G2P[4] | T* | N | S | N* | E | W | E | N | Q | D | T | M | N | K | Q | D | V | D* | N | N* | R | D | N | T | S | D | I | T* | G | ||
| RVA/Human-wt/BGN/J251/2010/G2P[4] | T* | N | S | N* | E | W | E | N | Q | D | T | M | N | K | Q | D | V | D* | N | N* | R | D | N | T | S | D | I | S | G | ||
| RVA/Human-wt/BGN/M334/2013/G2P[4] | T* | N | S | N* | E | W | E | N | Q | D | T | M | D* | K | Q | D | V | D* | N | N* | R | D | N | T | S | D | I | S | G | ||
| RVA/Human-wt/BGN/M315/2013/G2P[4] | T* | N | S | N* | E | W | E | N | Q | D | T | M | D* | K | Q | D | V | D* | N | N* | R | D | N | T | S | D | I | S | G | ||
| RVA/Human-wt/BGN/M313/2013/G2P[4] | T* | I* | S | N* | V* | W | E | N | Q | D | T | M | D* | K | Q | D | V | D* | N | N* | R | D | N | T | S | D | I | S | G | ||
| RVA/Human-wt/BGN/M312/2013/G2P[4] | T* | N | S | N* | E | W | E | N | Q | D | T | M | D* | K | Q | D | V | D* | N | N* | R | D | N | T | S | D | I | S | G | ||
| RVA/Human-wt/BGN/M310/2013/G2P[4] | T* | N | S | N* | E | W | E | N | Q | D | T | M | D* | K | Q | D | V | D* | N | N* | R | D | N | T | S | D | I | S | G | ||
| RVA/Human-wt/BGN/M292/2013/G2P[4] | T* | N | S | N* | E | W | E | N | Q | D | T | M | D* | K | Q | D | V | D* | N | N* | R | D | N | T | S | D | I | S | G | ||
| RVA/Human-wt/BGN/M289/2013/G2P[4] | T* | N | S | N* | E | W | E | N | Q | D | T | M | D* | K | Q | D | V | D* | N | N* | R | D | N | T | S | D | V* | A* | G | ||
| RVA/Human-wt/BGN/M282/2013/G2P[4] | T* | N | S | N* | E | W | E | N | Q | D | T | M | D* | K | Q | D | V | D* | N | N* | R | D | N | T | S | D | I | S | G | ||
Residues that differ from those of RotaTeq™ (G2 component).
Table 6a.
Alignment of the amino acid residues defining the neutralization domains of VP4, VP8 subunit (8–1, 8–2, 8–3 and 8–4) between the P[4] strains (DS-1, TB-Chen, MMC88) and 16 G2P[4] RVA strains in Bangladesh.
| RVA strain | 8-1 |
8-2 |
8-3 |
8-4 |
||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 100 | 146 | 148 | 150 | 188 | 190 | 192 | 193 | 194 | 195 | 196 | 180 | 183 | 113 | 114 | 115 | 116 | 125 | 131 | 132 | 133 | 135 | 87 | 88 | 89 | ||||
| RVA/Human-tc/USA/DS-1/1976/G2P[4] | D | S | H | D | S | T | D | L | N | N | I | T | A | S | Q | T | N | N | E | N | N | D | N | T | D | |||
| RVA/Human-tc/CHN/TB-Chen/1996/G2P[4] | D | S | Q | D | S | T | D | L | N | N | I | T | A | S | Q | T | N | N | E | N | N | D | N | T | N | |||
| RVA/Human-wt/BGN/MMC88/2005/G2P[4] | D | S | Q | D | S | T | D | L | N | N | I | T | A | S | Q | T | N | N | E | N | S* | D | N | T | D | |||
| RVA/Human-wt/BGN/J306/2010/G2P[4] | D | S | Q | D | S | T | D | L | N | N | I | T | A | S | Q | T | N | N | E | N | S* | D | N | T | D | |||
| RVA/Human-wt/BGN/J303/2010/G2P[4] | D | S | Q | D | S | T | D | L | N | N | I | T | A | S | Q | T | N | N | E | N | S* | D | N | T | D | |||
| RVA/Human-wt/BGN/J300/2010/G2P[4] | D | S | Q | D | S | T | D | L | N | N | I | T | A | S | Q | T | N | N | E | N | S* | D | N | T | D | |||
| RVA/Human-wt/BGN/J266/2010/G2P[4] | D | S | Q | D | S | T | D | L | N | N | I | T | A | S | Q | T | N | N | E | N | S* | D | N | T | D | |||
| RVA/Human-wt/BGN/J265/2010/G2P[4] | D | S | Q | D | S | T | D | L | N | N | I | T | A | S | Q | T | N | N | E | N | S* | D | N | T | D | |||
| RVA/Human-wt/BGN/J263/2010/G2P[4] | D | S | Q | D | S | T | D | L | N | N | I | T | A | S | Q | T | N | N | E | N | S* | D | N | T | D | |||
| RVA/Human-wt/BGN/J253/2010/G2P[4] | D | S | Q | D | S | T | D | L | N | N | I | T | A | S | Q | T | N | N | E | N | S* | D | N | T | D | |||
| RVA/Human-wt/BGN/J251/2010/G2P[4] | D | S | Q | D | S | T | D | L | N | N | I | T | A | S | Q | T | N | N | E | N | S* | D | N | T | D | |||
| RVA/Human-wt/BGN/M334/2013/G2P[4] | D | S | Q | D | S | T | D | L | N | N | I | T | A | S | P* | T | N | N | E | N | S* | D | N | A* | D | |||
| RVA/Human-wt/BGN/M315/2013/G2P[4] | D | S | Q | D | S | T | D | L | N | N | I | T | A | S | P* | T | N | N | E | N | S* | D | N | A* | D | |||
| RVA/Human-wt/BGN/M313/2013/G2P[4] | D | S | Q | D | S | T | D | L | N | N | I | T | A | S | P* | T | N | N | E | N | S* | D | N | A* | D | |||
| RVA/Human-wt/BGN/M312/2013/G2P[4] | D | S | Q | D | S | T | D | L | N | N | I | T | A | S | P* | T | N | N | E | N | S* | D | N | A* | D | |||
| RVA/Human-wt/BGN/M310/2013/G2P[4] | D | S | Q | D | S | T | D | L | N | N | I | T | A | S | P* | T | N | N | E | N | S* | D | N | A* | D | |||
| RVA/Human-wt/BGN/M292/2013/G2P[4] | D | S | Q | D | S | T | D | L | N | N | I | T | A | S | P* | T | N | N | E | N | S* | D | N | A* | D | |||
| RVA/Human-wt/BGN/M289/2013/G2P[4] | D | S | Q | D | S | T | D | L | N | N | I | T | A | S | P* | T | N | N | E | N | S* | D | N | A* | D | |||
| RVA/Human-wt/BGN/M282/2013/G2P[4] | D | S | Q | D | S | T | D | L | N | N | I | T | A | S | P* | T | N | N | E | N | S* | D | N | A* | D | |||
Residues that differ from those of strains DS-1 and TB-Chen.
Table 6b.
Alignment of the amino acid residues defining the neutralization domains of VP4, VP5 subunit (5–1, 5–2, 5–3, 5–4 and 5–5) between the P[4] strains (DS-1, TB-Chen, MMC88) and 16 G2P[4] RVA strains in Bangladesh.
| RVA strain | 5-1 |
5-2 |
5-3 |
5-4 |
5-5 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 384 | 386 | 388 | 393 | 394 | 398 | 440 | 441 | 434 | 459 | 429 | 306 | |||||
| RVA/Human-tc/USA/DS-1/1976/G2P[4] | Y | F | L | W | P | G | R | T | P | T | L | R | ||||
| RVA/Human-tc/CHN/TB-Chen/1996/G2P[4] | Y | F | L | W | P | G | R | T | P | T | L | R | ||||
| RVA/Human-wt/BGN/MMC88/2005/G2P[4] | Y | F | L | W | P | G | R | T | P | T | L | R | ||||
| RVA/Human-wt/BGN/J306/2010/G2P[4] | Y | F | L | W | P | G | R | T | P | T | L | R | ||||
| RVA/Human-wt/BGN/J303/2010/G2P[4] | Y | F | L | W | P | G | R | T | P | T | L | R | ||||
| RVA/Human-wt/BGN/J300/2010/G2P[4] | Y | F | L | W | P | G | R | T | P | T | L | R | ||||
| RVA/Human-wt/BGN/J266/2010/G2P[4] | Y | F | L | W | P | G | R | T | P | T | L | R | ||||
| RVA/Human-wt/BGN/J265/2010/G2P[4] | Y | F | L | W | P | G | R | T | P | T | L | R | ||||
| RVA/Human-wt/BGN/J263/2010/G2P[4] | Y | F | L | W | P | G | R | T | P | T | L | R | ||||
| RVA/Human-wt/BGN/J253/2010/G2P[4] | Y | F | L | W | P | G | R | T | P | T | L | R | ||||
| RVA/Human-wt/BGN/J251/2010/G2P[4] | Y | F | L | W | P | G | R | T | P | T | L | R | ||||
| RVA/Human-wt/BGN/M334/2013/G2P[4] | Y | F | L | W | P | G | R | T | P | T | L | R | ||||
| RVA/Human-wt/BGN/M315/2013/G2P[4] | Y | F | L | W | P | G | R | T | P | T | L | R | ||||
| RVA/Human-wt/BGN/M313/2013/G2P[4] | Y | F | L | W | P | G | R | T | P | T | L | R | ||||
| RVA/Human-wt/BGN/M312/2013/G2P[4] | Y | F | L | W | P | G | R | T | P | T | L | R | ||||
| RVA/Human-wt/BGN/M310/2013/G2P[4] | Y | F | L | W | P | G | R | T | P | T | L | R | ||||
| RVA/Human-wt/BGN/M292/2013/G2P[4] | Y | F | L | W | P | G | R | T | P | T | L | R | ||||
| RVA/Human-wt/BGN/M289/2013/G2P[4] | Y | F | L | W | P | G | R | T | P | T | L | R | ||||
| RVA/Human-wt/BGN/M282/2013/G2P[4] | Y | F | L | W | P | G | R | T | P | T | L | R | ||||
4. Discussion
Recent molecular epidemiological studies of whole genome of G2P[4] HRVs for a long period conducted in Italy (Giammanco et al., 2014), and Japan (Doan et al., 2015) indicated that G2P[4] strains distributed globally appeared to have undergone intragenotype reassortment, observed by change of lineages of all the viral protein genes from the 1970s until 2011. As a result, it was suggested that major changes of genomic composition of the G2P[4] HRVs might occur in the early 2000s, when the “new” global G2P[4] HRV strains have been widespread replacing the “old” G2P[4] strains. In the present study, all the 16 RVA strains in 2010 and 2013 in Bangladesh belonged to the group of “new” G2P[4] HRVs clustering with recent global G2P[4] strains. This view was supported by the fact that amino acid residues in the VP7 antigenic regions were the same as those found in recent global G2P[4] HRVs, distinguishing them from old strains as well as the G2 component of pentavalent vaccine (Afrad et al., 2014; Dennis et al., 2014; Doan et al., 2011; Donato et al., 2014; Gómez et al., 2014; Zeller et al., 2011).
It was noted in the present study that change and replacement of sublineages and lineage constellations were observed for a short period of 3 years, although all the gene segments exhibited high sequence identities to each other. Individual viral genome segments were differentiated into 1–3 sublineages in a single lineage of individual genotypes (2 lineages in M2-VP3 and 3 lineages in E2-NSP4 genes). Thereby the 16 Bangladeshi strains were classified into four clades containing 8 lineage constellations, revealing the presence of three clades with four lineage constellations in 2010, and one clade with four constellations in 2013. Therefore, co-existence of multiple G2P[4] HRV strains with different lineage constellations was demonstrated in Bangladesh in the present study. Using classification of lineage (not sublineage as in the present study) of rotavirus genes, different allele constellations have been identified for G2P[4] HRV in a single winter season in a US community (Dennis et al., 2014), and also for G1, G3, and G4 RVAs in different study settings worldwide (McDonald et al., 2009; McDonald et al., 2011; McDonald et al., 2012; Wang et al., 2014).
Through analysis of VP7 from a number of G2 strains in the last 39 years in the US, lineage turnover was presumed to occur every 7 years on an average, generating new dominant strains (Dennis et al., 2014). Afrad and coworkers revealed that multiple lineages of G1 and G2HRVs co-circulated for one or a few seasons, followed by frequent replacement with different lineages, by the analysis of the VP7 gene of HRVs detected for more than 20 years in Bangladesh (Afrad et al., 2014). Therefore, lineage of the G2-VP7 gene is considered to change frequently in global level, as suggested by another study in Australia (Donato et al., 2014). Our present study, despite a short period, change of sublineages was documented for a whole genome, providing more detailed evidences than lineage.
In our study in Bangladesh, lineage groups (clade A-C) in 2010 were considered to be replaced by clade D in 2013. However, clade A had VP1, VP3, and NSP4 genes belonging to sublineage Va which was commonly found in clade D. Therefore, it is suggested that clade A HRVs in 2010 were one of the ancestral viruses to generate clade D HRVs in 2013. Viral genome segments of clade D HRV were mostly assigned to sublineage Va, which was unique to present Bangladeshi strains in 2013 without clustering with other global strains. This finding suggested that strains of clade D may be newly emerging G2P[4] HRVs in Bangladesh. Furthermore, VP4 of clade D HRVs characteristically possess alanine and proline at position 88 and 114 in the antigenic region, respectively. Both amino acids are not found in Bangladeshi strain MMC88 as well as global G2P[4] strains (Giammanco et al., 2014). Proline at position 114 is conserved in P[8] VP4, and also identified in only a few G2P[4] Brazilian strains in 2010 (Gómez et al., 2014). It is therefore important to survey global spread and distribution of the G2P[4] RVA genes belonging to the clade D, and their antigenic characteristics of VP4. In contrast, clade C strains that were detected in only 2010 had asparagine at position 130 of VP7, like strains DS-1 and TB-Chen, suggesting that these strains have a trait of old G2HRVs.
VP3 gene of a Bangladeshi strain MMC88 in 2005 clustered with most G2P[4] HRV strains in the sublineage Va. The MMC88-VP3 gene is genetically related to caprine rotavirus strain GO34 from Bangladesh and suggests an animal origin (Ghosh et al., 2010a; Ghosh et al., 2011b), while another G2P[4] strain, MMC6, detected in 2005 has a VP3 gene frequently detected in HRVs. It was interesting in the present study that such caprine-like VP3 gene was identified in most of G2P[4] strains in 2010 and 2013 (lineage Va), suggesting successful adaptation of this gene to Bangladeshi HRVs of DS-1 genogroup. This may be evidenced by the fact that the VP3 genes clustering with the caprine strain and MMC88 have been detected in USA, Brazil, Thailand, Australia, and Italy (Dennis et al., 2014; Giammanco et al., 2014; Gómez et al., 2014).
In conclusion, our present study elucidated that multiple, genetically distinct G2P[4] HRVs are circulating in north-central Bangladesh, by whole genome-based phylogenetic analysis. Replacement of genomic constellations, and amino acid change in the antigenic region in VP4 were observed even for a short period from 2010 to 2013. Further continuous surveillance of G2P[4] HRVs is necessary at a global level to understand their evolutionary state.
Declarations
Author contribution statement
Satoru Aida, Nobumichi Kobayashi: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Samsoon Nahar, Shyamal K. Paul, Muhammad A. Hossain, Muhammad R. Kabir, Santana R. Sarkar, Salma Ahmed, Souvik Ghosh, Meiji S. Aung: Performed the experiments.
Noriko Urushibara, Mitsuyo Kawaguchiya, Ayako Sumi: Contributed reagents, materials, analysis tools or data.
Funding statement
Nobumichi Kobayashi was supported by the Grant-in-Aid for Scientific Research (Grant no. 25305022) from the Japan Society for the Promotion of Science.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Afrad M.H., Hassan Z., Farjana S., Moni S., Barua S., Das S.K., Faruque A.S., Azim T., Rahman M. Changing profile of rotavirus genotypes in Bangladesh, 2006-2012. BMC Infect. Dis. 2013;13(320) doi: 10.1186/1471-2334-13-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afrad M.H., Matthijnssens J., Afroz S.F., Rudra P., Nahar L., Rahman R., Hossain M.E., Rahman S.R., Azim T., Rahman M. Differences in lineage replacement dynamics of G1 and G2 rotavirus strains versus G9 strain over a period of 22 years in Bangladesh. Infect. Genet. Evol. 2014;28:214–222. doi: 10.1016/j.meegid.2014.10.002. [DOI] [PubMed] [Google Scholar]
- Ahmed S., Hussain M., Akhter S., Islam T., Ahmed S.U., Kabir M.L. Genotypes of rotavirus diarrhoea in a children hospital of Bangladesh. Mymensingh Med. J. 2012;21:497–502. [PubMed] [Google Scholar]
- Bányai K., Mijatovic-Rustempasic S., Hull J.J., Esona M.D., Freeman M.M., Frace A.M., Bowen M.D., Gentsch J.R. Sequencing and phylogenetic analysis of the coding region of six common rotavirus strains: evidence for intragenogroup reassortment among co-circulating G1P[8] and G2P[4] strains from the United States. J. Med. Virol. 2011;83:532–539. doi: 10.1002/jmv.21977. [DOI] [PubMed] [Google Scholar]
- Chaimongkol N., Khamrin P., Malasao R., Thongprachum A., Ushijima H., Maneekarn N. Genotypic linkages of gene segments of rotaviruses circulating in pediatric patients with acute gastroenteritis in Thailand. Infect. Genet. Evol. 2012;12:1381–1391. doi: 10.1016/j.meegid.2012.04.015. [DOI] [PubMed] [Google Scholar]
- Dennis A.F., McDonald S.M., Payne D.C., Mijatovic-Rustempasic S., Esona M.D., Edwards K.M., Chappell J.D., Patton J.T. Molecular epidemiology of contemporary G2P[4] human rotaviruses cocirculating in a single U S. community: footprints of a globally transitioning genotype. J. Virol. 2014;88:3789–3801. doi: 10.1128/JVI.03516-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey S.K., Hayakawa Y., Rahman M., Islam R., Mizuguchi M., Okitsu S., Ushijima H. G2 strain of rotavirus among infants and children Bangladesh. Emerg. Infect. Dis. 2009;15:91–94. doi: 10.3201/eid1501.080883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doan Y.H., Nakagomi T., Cunliffe N.A., Pandey B.D., Sherchand J.B., Nakagomi O. The occurrence of amino acid substitutions D96 N and S242 N in VP7 of emergent G2P[4] rotaviruses in Nepal in 2004-2005: a global and evolutionary perspective. Arch. Virol. 2011;156:1969–1978. doi: 10.1007/s00705-011-1083-z. [DOI] [PubMed] [Google Scholar]
- Doan Y.H., Nakagomi T., Agbemabiese C.A., Nakagomi O. Changes in the distribution of lineage constellations of G2P[4] Rotavirus A strains detected in Japan over 32 years (1980-2011) Infect. Genet. Evol. 2015;34:423–433. doi: 10.1016/j.meegid.2015.05.026. [DOI] [PubMed] [Google Scholar]
- Donato C.M., Zhang Z.A., Donker N.C., Kirkwood C.D. Characterization of G2P[4] rotavirus strains associated with increased detection in Australian states using the RotaTeq® vaccine during the 2010-2011 surveillance period. Infect. Genet. Evol. 2014;28:398–412. doi: 10.1016/j.meegid.2014.05.020. [DOI] [PubMed] [Google Scholar]
- Dóró R., László B., Martella V., Leshem E., Gentsch J., Parashar U., Bányai K. Review of global rotavirus strain prevalence data from six years post vaccine licensure surveillance: is there evidence of strain selection from vaccine pressure? Infect. Genet. Evol. 2014;28:446–461. doi: 10.1016/j.meegid.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M.K., Greenberg H.B. Rotaviruses. In: Knipe D.M., Howley P.M., Cohen J.I., Griffin D.E., Lamb R.A., Martin M.A., Racaniello V.R., Roizman B., editors. Fields Virology, 6th edn. Lippincott Williams & Wilkins; Philadelphia: 2013. pp. 1347–1401. [Google Scholar]
- Ghosh S., Alam M.M., Ahmed M.U., Talukdar R.I., Paul S.K., Kobayashi N. Complete genome constellation of a caprine group A rotavirus strain reveals common evolution with ruminant and human rotavirus strains. J. Gen. Virol. 2010;91(Pt 9):2367–2373. doi: 10.1099/vir.0.022244-0. [DOI] [PubMed] [Google Scholar]
- Ghosh S., Kobayashi N., Nagashima S., Chawla-Sarkar M., Krishnan T., Ganesh B., Naik T. Full genomic analysis and possible origin of a porcine G12 rotavirus strain RU172. Virus Genes. 2010;40:382–388. doi: 10.1007/s11262-010-0454-y. [DOI] [PubMed] [Google Scholar]
- Ghosh S., Kobayashi N. Whole genomic analysis of rotavirus strains: current status and future prospects. Future Microbiol. 2011;6:1049–1065. doi: 10.2217/fmb.11.90. [DOI] [PubMed] [Google Scholar]
- Ghosh S., Paul S.K., Hossain M.A., Alam M.M., Ahmed M.U., Kobayashi N. Full genomic analyses of two human G2P[4] rotavirus strains detected in 2005: identification of a caprine-like VP3 gene. J. Gen. Virol. 2011;92(Pt 5):1222–1227. doi: 10.1099/vir.0.029868-0. [DOI] [PubMed] [Google Scholar]
- Giammanco G.M., Bonura F., Zeller M., Heylen E., Van Ranst M., Martella V., Bányai K., Matthijnssens J., De Grazia S. Evolution of DS-1-like human G2P[4] rotaviruses assessed by complete genome analyses. J. Gen. Virol. 2014;95(Pt 1):91–109. doi: 10.1099/vir.0.056788-0. [DOI] [PubMed] [Google Scholar]
- Gómez M.M., Carvalho-Costa F.A., Volotão Ede M., Rose T.L., da Silva M.F., Fialho A.M., Assis R.M., de Andrade Jda S., Sá A.C., Zeller M., Heylen E., Matthijnssens J., Leite J.P. Prevalence and genomic characterization of G2P[4] group A rotavirus strains during monovalent vaccine introduction in Brazil. Infect. Genet. Evol. 2014;28:486–494. doi: 10.1016/j.meegid.2014.09.012. [DOI] [PubMed] [Google Scholar]
- Greenberg H.B., Kalica A.R., Wyatt R.G., Jones R.W., Kapikian A.Z., Chanock R.M. Rescue of noncultivatable human rotavirus by gene reassortment during mixed infection with ts mutants of a cultivatable bovine rotavirus. Proc. Natl. Acad. Sci. U S A. 1981;78:420–424. doi: 10.1073/pnas.78.1.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurgel R.Q., Alvarez A., de J., Rodrigues A., Ribeiro R.R., Dolabella S.S., Da Mota N.L., Santos V.S., Iturriza-Gomara M., Cunliffe N.A., Cuevas L.E. Incidence of rotavirus and circulating genotypes in Northeast Brazil during 7 years of national rotavirus vaccination. PLoS One. 2014;9 doi: 10.1371/journal.pone.0110217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iturriza-Gómara M., Kang G., Gray J. Rotavirus genotyping: keeping up with an evolving population of human rotaviruses. J. Clin. Virol. 2004;31:259–265. doi: 10.1016/j.jcv.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Kim J.S., Kim H.S., Hyun J., Kim H.S., Song W., Lee K.M., Shin S.H. Analysis of rotavirus genotypes in Korea during 2013: an increase in the G2P[4] genotype after the introduction of rotavirus vaccines. Vaccine. 2014;32:6396–6402. doi: 10.1016/j.vaccine.2014.09.067. [DOI] [PubMed] [Google Scholar]
- Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- Kirkwood C.D., Boniface K., Bishop R.F., Barnes G.L. Australian Rotavirus Surveillance Group, 2009. Australian Rotavirus Surveillance Program annual report, 2008/2009. Commun. Dis. Intell. Q. Rep. 2009;33:382–388. [PubMed] [Google Scholar]
- Kirkwood C.D., Roczo S., Boniface K., Bishop R.F., Barnes G.L. Australian Rotavirus Surveillance Group, 2011. Australian Rotavirus Surveillance Program annual report, 2010/2011. Commun. Dis. Intell. Q. Rep. 2010;35:281–287. [PubMed] [Google Scholar]
- Lanata C.F., Fischer-Walker C.L., Olascoaga A.C., Torres C.X., Aryee M.J., Black R.E. Child Health Epidemiology Reference Group of the World Health Organization and UNICEF. 2013. Global causes of diarrheal disease mortality in children <5 years of age: a systematic review. PLoS One. 2013;8 doi: 10.1371/journal.pone.0072788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandile M.G., Esteban L.E., Argüelles M.H., Mistchenko A., Glikmann G., Castello A.A. Surveillance of group A Rotavirus in Buenos Aires 2008-2011, long lasting circulation of G2P[4] strains possibly linked to massive monovalent vaccination in the region. J. Clin. Virol. 2014;60:282–289. doi: 10.1016/j.jcv.2014.04.022. [DOI] [PubMed] [Google Scholar]
- Matthijnssens J., Ciarlet M., Heiman E., Arijs I., Delbeke T., McDonald S.M., Palombo E.A., Iturriza-Gómara M., Maes P., Patton J.T., Rahman M., Van Ranst M. Full genome-based classification of rotaviruses reveals a common origin between human Wa-Like and porcine rotavirus strains and human DS-1-like and bovine rotavirus strains. J. Virol. 2008;82:3204–3219. doi: 10.1128/JVI.02257-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthijnssens J., Ciarlet M., McDonald S.M., Attoui H., Bányai K., Brister J.R., Buesa J., Esona M.D., Estes M.K., Gentsch J.R., Iturriza-Gómara M., Johne R., Kirkwood C.D., Martella V., Mertens P.P., Nakagomi O., Parreño V., Rahman M., Ruggeri F.M., Saif L.J., Santos N., Steyer A., Taniguchi K., Patton J.T., Desselberger U., Van Ranst M. Uniformity of rotavirus strain nomenclature proposed by the Rotavirus Classification Working Group (RCWG) Arch. Virol. 2011;156:1397–1413. doi: 10.1007/s00705-011-1006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthijnssens J., Zeller M., Heylen E., De Coster S., Vercauteren J., Braeckman T., Van Herck K., Meyer N., Pirçon J.Y., Soriano-Gabarro M., Azou M., Capiau H., De Koster J., Maernoudt A.S., Raes M., Verdonck L., Verghote M., Vergison A., Van Damme P., Van Ranst M. RotaBel study group, 2014. Higher proportion of G2P[4] rotaviruses in vaccinated hospitalized cases compared with unvaccinated hospitalized cases, despite high vaccine effectiveness against heterotypic G2P[4] rotaviruses. Clin. Microbiol. Infect. 2014;20:O702–O710. doi: 10.1111/1469-0691.12612. [DOI] [PubMed] [Google Scholar]
- McDonald S.M., Matthijnssens J., McAllen J.K., Hine E., Overton L., Wang S., Lemey P., Zeller M., Van Ranst M., Spiro D.J., Patton J.T. Evolutionary dynamics of human rotaviruses: balancing reassortment with preferred genome constellations. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald S.M., Davis K., McAllen J.K., Spiro D.J., Patton J.T. Intra-genotypic diversity of archival G4P[8] human rotaviruses from Washington. DC. Infect. Genet. Evol. 2011;11:1586–1594. doi: 10.1016/j.meegid.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald S.M., McKell A.O., Rippinger C.M., McAllen J.K., Akopov A., Kirkness E.F., Payne D.C., Edwards K.M., Chappell J.D., Patton J.T. Diversity and relationships of cocirculating modern human rotaviruses revealed using large-scale comparative genomics. J. Virol. 2012;86:9148–9162. doi: 10.1128/JVI.01105-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midthun K., Valdesuso J., Hoshino Y., Flores J., Kapikian A.Z., Chanock R.M. Analysis by RNA-RNA hybridization assay of intertypic rotaviruses suggests that gene reassortment occurs in vivo. J. Clin. Microbiol. 1987;25:295–300. doi: 10.1128/jcm.25.2.295-300.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles M.G., Lewis K.D., Kang G., Parashar U.D., Steele A.D. A systematic review of rotavirus strain diversity in India, Bangladesh, and Pakistan. Vaccine. 2012;30(Suppl 1):A131–A139. doi: 10.1016/j.vaccine.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Mullick S., Mandal P., Nayak M.K., Ghosh S., De P Rajendran K., Bhattacharya M.K., Mitra U., Ramamurthy T., Kobayashi N., Chawla-Sarkar M. Hospital based surveillance and genetic characterization of rotavirus strains in children (<5 years) with acute gastroenteritis in Kolkata, India, revealed resurgence of G9 and G2 genotypes during 2011-2013. Vaccine. 2014;32(Suppl 1):A20–A28. doi: 10.1016/j.vaccine.2014.03.018. [DOI] [PubMed] [Google Scholar]
- Nagashima S., Kobayashi N., Paul S.K., Ghosh S., Chawla-Sarkar M., Hossain M.A., Krishnan T. Identification of P[8]b subtype in OP354-like human rotavirus strains by a modified RT-PCR method. Jpn. J. Infect. Dis. 2010;63:208–211. [PubMed] [Google Scholar]
- Page N.A., Steele A.D. Antigenic and genetic characterization of serotype G2 human rotavirus strains from the African continent. J. Clin. Microbiol. 2004;42:595–600. doi: 10.1128/JCM.42.2.595-600.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S.K., Kobayashi N., Nagashima S., Ishino M., Watanabe S., Alam M.M., Ahmed M.U., Hossain M.A., Naik T.N. Phylogenetic analysis of rotaviruses with genotypes G1 G2, G9 and G12 in Bangladesh: evidence for a close relationship between rotaviruses from children and adults. Arch. Virol. 2008;153:1999–2012. doi: 10.1007/s00705-008-0212-9. [DOI] [PubMed] [Google Scholar]
- Rahman M., Matthijnssens J., Yang X., Delbeke T., Arijs I., Taniguchi K., Iturriza-Gómara M., Iftekharuddin N., Azim T., Van Ranst M. Evolutionary history and global spread of the emerging g12 human rotaviruses. J. Virol. 2007;81:2382–2390. doi: 10.1128/JVI.01622-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M., Matthijnssens J., Saiada F., Hassan Z., Heylen E., Azim T., Van Ranst M. Complete genomic analysis of a Bangladeshi G1P[8] rotavirus strain detected in 2003 reveals a close evolutionary relationship with contemporary human Wa-like strains. Infect. Genet. Evol. 2010;10:746–754. doi: 10.1016/j.meegid.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Santos N., Hoshino Y. Global distribution of rotavirus serotypes/genotypes and its implication for the development and implementation of an effective rotavirus vaccine. Rev. Med. Virol. 2005;15:29–56. doi: 10.1002/rmv.448. [DOI] [PubMed] [Google Scholar]
- Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojnar E., Sachsenröder J., Twardziok S., Reetz J., Otto P.H., Johne R. Identification of an avian group A rotavirus containing a novel VP4 gene with a close relationship to those of mammalian rotaviruses. J. Gen. Virol. 2013;94:136–142. doi: 10.1099/vir.0.047381-0. [DOI] [PubMed] [Google Scholar]
- Urasawa S., Urasawa T., Taniguchi K. Genetic reassortment between two human rotaviruses having different serotype and subgroup specificities. J. Gen. Virol. 1986;67:1551–1559. doi: 10.1099/0022-1317-67-8-1551. [DOI] [PubMed] [Google Scholar]
- Wang Y.H., Pang B.B., Ghosh S., Zhou X., Shintani T., Urushibara N., Song Y.W., He M.Y., Liu M.Q., Tang W.F., Peng J.S., Hu Q., Zhou D.J., Kobayashi N. Molecular epidemiology and genetic evolution of the whole genome of G3P[8] human rotavirus in Wuhan, China, from 2000 through 2013. PLoS One. 2014;9 doi: 10.1371/journal.pone.0088850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller M., Patton J.T., Heylen E., De Coster S., Ciarlet M., Van Ranst M., Matthijnssens J. Genetic analyses reveal differences in the VP7 and VP4 antigenic epitopes between human rotaviruses circulating in Belgium and rotaviruses in Rotarix and RotaTeq. J. Clin. Microbiol. 2011;50:966–976. doi: 10.1128/JCM.05590-11. [DOI] [PMC free article] [PubMed] [Google Scholar]