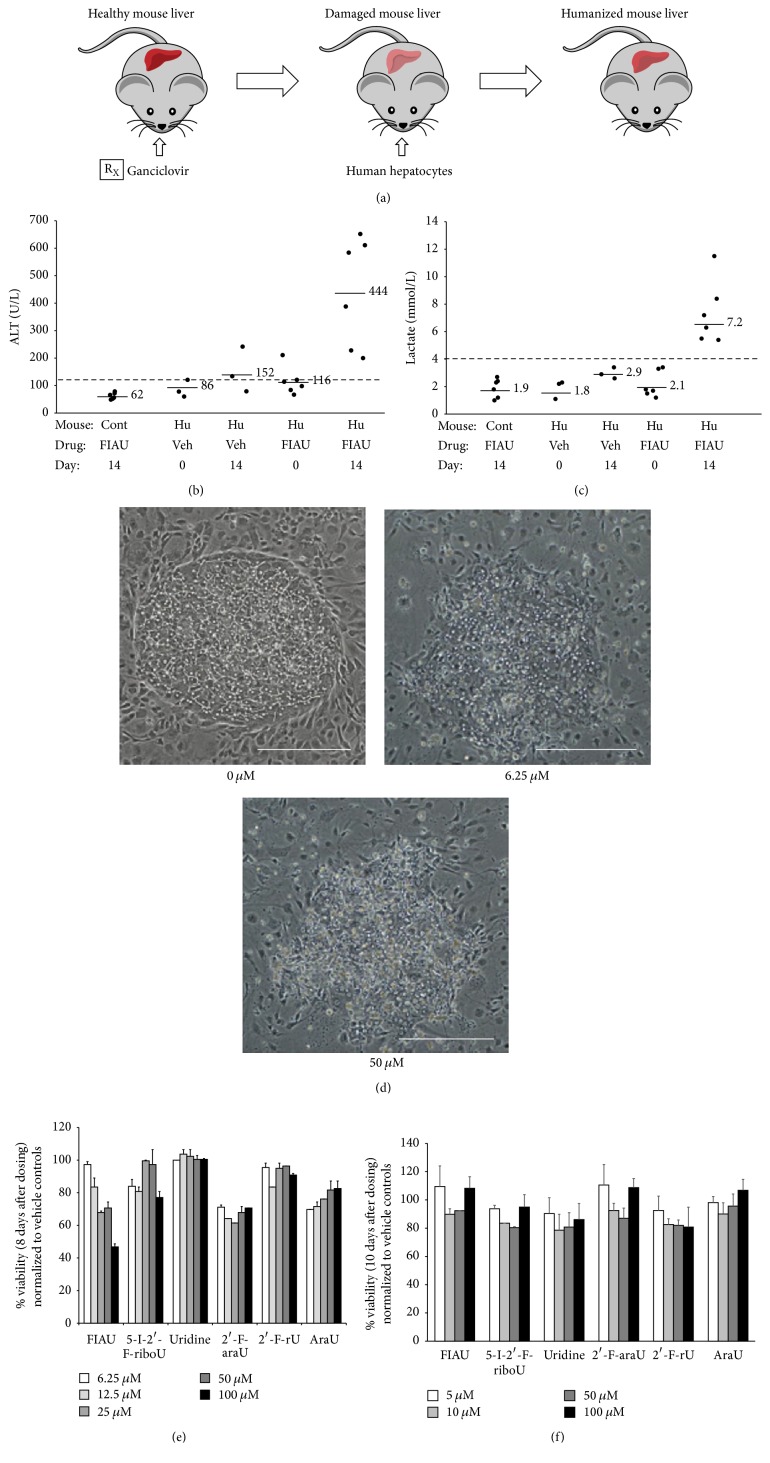
Figure 2.
Fialuridine toxicity assessment in humanized rodents and micropatterned cocultures (MPCCs) containing either primary human hepatocytes (PHH) or primary rat hepatocytes. Mice were briefly exposed to a nontoxic dose of ganciclovir to ablate murine liver cells (panel (a)) [118]. PHHs were transplanted into 8-week-old mice and the humanized liver was established for 8 weeks prior to toxicology studies. Humanized and control nonhumanized mice were dosed with vehicle (0.5% dimethylsulfoxide) or 2.5 mg/kg/d fialuridine for 14 days by oral gavage. Plasma alanine aminotransferase or ALT (panel (b)) and lactate (panel (c)) levels were measured on days 0 and 14 [78]. Each dot in the graphs of panels (b) and (c) represents 1 mouse, the solid lines adjacent to the dots represent averages for each sample group, and the dashed line across each graph represents the upper limit of normal. PHH-MPCCs were dosed for 8 days with 0, 6.25, or 50 µM fialuridine and deteriorating hepatocyte morphology was recorded with increasing dose (panel (d)) [20]. Scale bars on images represent ~250 µm. In addition to fialuridine (5-I-2′-F-araU), PHH-MPCCs were dosed for 8 days with several doses of 5 other analog compounds. Mitochondrial activity was assessed using the MTT assay and normalized to vehicle only controls (panel (e)). Only fialuridine caused a dose-dependent toxicity in PHH-MPCCs. On the other hand, no dose-dependent toxicity was observed in MPCCs created using primary rat hepatocytes and dosed with the compounds for 8 days (panel (f)).